Effect of Helmet Noninvasive Ventilation vs High-Flow Nasal Oxygen on Days Free of Respiratory Support in Patients With COVID-19 and Moderate to Severe Hypoxemic Respiratory Failure: The HENIVOT Randomized Clinical Trial
- PMID: 33764378
- PMCID: PMC7995134
- DOI: 10.1001/jama.2021.4682
Effect of Helmet Noninvasive Ventilation vs High-Flow Nasal Oxygen on Days Free of Respiratory Support in Patients With COVID-19 and Moderate to Severe Hypoxemic Respiratory Failure: The HENIVOT Randomized Clinical Trial
Abstract
Importance: High-flow nasal oxygen is recommended as initial treatment for acute hypoxemic respiratory failure and is widely applied in patients with COVID-19.
Objective: To assess whether helmet noninvasive ventilation can increase the days free of respiratory support in patients with COVID-19 compared with high-flow nasal oxygen alone.
Design, setting, and participants: Multicenter randomized clinical trial in 4 intensive care units (ICUs) in Italy between October and December 2020, end of follow-up February 11, 2021, including 109 patients with COVID-19 and moderate to severe hypoxemic respiratory failure (ratio of partial pressure of arterial oxygen to fraction of inspired oxygen ≤200).
Interventions: Participants were randomly assigned to receive continuous treatment with helmet noninvasive ventilation (positive end-expiratory pressure, 10-12 cm H2O; pressure support, 10-12 cm H2O) for at least 48 hours eventually followed by high-flow nasal oxygen (n = 54) or high-flow oxygen alone (60 L/min) (n = 55).
Main outcomes and measures: The primary outcome was the number of days free of respiratory support within 28 days after enrollment. Secondary outcomes included the proportion of patients who required endotracheal intubation within 28 days from study enrollment, the number of days free of invasive mechanical ventilation at day 28, the number of days free of invasive mechanical ventilation at day 60, in-ICU mortality, in-hospital mortality, 28-day mortality, 60-day mortality, ICU length of stay, and hospital length of stay.
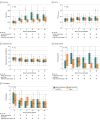
Results: Among 110 patients who were randomized, 109 (99%) completed the trial (median age, 65 years [interquartile range {IQR}, 55-70]; 21 women [19%]). The median days free of respiratory support within 28 days after randomization were 20 (IQR, 0-25) in the helmet group and 18 (IQR, 0-22) in the high-flow nasal oxygen group, a difference that was not statistically significant (mean difference, 2 days [95% CI, -2 to 6]; P = .26). Of 9 prespecified secondary outcomes reported, 7 showed no significant difference. The rate of endotracheal intubation was significantly lower in the helmet group than in the high-flow nasal oxygen group (30% vs 51%; difference, -21% [95% CI, -38% to -3%]; P = .03). The median number of days free of invasive mechanical ventilation within 28 days was significantly higher in the helmet group than in the high-flow nasal oxygen group (28 [IQR, 13-28] vs 25 [IQR 4-28]; mean difference, 3 days [95% CI, 0-7]; P = .04). The rate of in-hospital mortality was 24% in the helmet group and 25% in the high-flow nasal oxygen group (absolute difference, -1% [95% CI, -17% to 15%]; P > .99).
Conclusions and relevance: Among patients with COVID-19 and moderate to severe hypoxemia, treatment with helmet noninvasive ventilation, compared with high-flow nasal oxygen, resulted in no significant difference in the number of days free of respiratory support within 28 days. Further research is warranted to determine effects on other outcomes, including the need for endotracheal intubation.
Trial registration: ClinicalTrials.gov Identifier: NCT04502576.
Conflict of interest statement
Figures


Comment in
-
Respiratory Support During the COVID-19 Pandemic: Is It Time to Consider Using a Helmet?JAMA. 2021 May 4;325(17):1723-1725. doi: 10.1001/jama.2021.4975. JAMA. 2021. PMID: 33764370 No abstract available.
-
[Focus ventilation, oxygen therapy and weaning : Intensive medical care studies from 2020/2021].Anaesthesist. 2021 Nov;70(11):967-976. doi: 10.1007/s00101-021-00979-8. Epub 2021 Oct 6. Anaesthesist. 2021. PMID: 34613457 Free PMC article. German. No abstract available.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

