Evolution of the Randomized Clinical Trial in the Era of Precision Oncology
- PMID: 33764385
- PMCID: PMC7995135
- DOI: 10.1001/jamaoncol.2021.0379
Evolution of the Randomized Clinical Trial in the Era of Precision Oncology
Abstract
Importance: The randomized clinical trial (RCT) in oncology has evolved since its widespread adoption in the 1970s. In recent years, concerns have emerged regarding the use of putative surrogate end points, such as progression-free survival (PFS), and marginal effect sizes.
Objective: To describe contemporary trends in oncology RCTs and compare these findings with earlier eras of RCT design and output.
Design, setting, and participants: Retrospective cohort study of systemic therapy RCTs in breast, colorectal, and non-small cell lung cancer published in 7 major journals between 2010 and 2020. This strategy replicates prior work and allows for comparison of trends with RCTs published between 1995 to 2004 and 2005 to 2009.
Main outcomes and measures: Data on RCT design, funding, results, and reporting were extracted from the published RCT report. Findings from the current period (2010-2020) were compared with data from RCTs published from 1995 to 2004 and 2005 to 2009. Descriptive and bivariate statistics were used to analyze temporal trends.
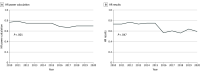
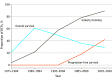
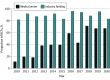
Results: The cohort included 298 RCTs (132 [44%] breast, 111 [37%] non-small cell lung cancer, 55 [19%] colorectal cancer). Experimental treatment included molecular inhibitor (171 of 298 [57%]), cytotoxic (83 of 298 [28%]), hormone (15 of 298 [5%]), and immune (24 of 298 [8%]) therapies. Sixty-nine percent (206 of 298) of RCTs were of palliative intent. The most common primary end point is now PFS; this has increased substantially over time (from 0% [0 of 167] to 18% [25 of 137] to 42% [125 of 298]; P < .001). Of 298 RCTs, 265 (89%) are now funded by industry (previously 95 of 167 [57%] and 107 of 137 [78%]; P < .001). Fifty-eight percent (173 of 298) of trials met their primary end point. Among positive trials, median improvement in overall survival and PFS was 3.4 and 2.9 months, respectively. More than one-third (117 of 298 [39%]) of reports used a professional medical writer; this increased substantially during the study period (from 3 of 27 [11%] in 2010 to 12 of 18 [67%] in 2020; P < .001).
Conclusions and relevance: This cohort study suggests that contemporary oncology RCTs now largely measure putative surrogate end points and are almost exclusively funded by the pharmaceutical industry. The increasing role of medical writers warrants attention. To demonstrate that new cancer treatments are high value, the oncology community needs to consider the extent to which study end points and target effect size provide meaningful benefit to patients.
Conflict of interest statement
Figures
Comment in
-
Randomized Clinical Trials in the Era of Precision Oncology-The Role of End Points, Industry Funding, and Medical Writing Integrity-Reply.JAMA Oncol. 2021 Oct 1;7(10):1579-1580. doi: 10.1001/jamaoncol.2021.3344. JAMA Oncol. 2021. PMID: 34436516 No abstract available.
-
Randomized Clinical Trials in the Era of Precision Oncology-The Role of End Points, Industry Funding, and Medical Writing Integrity.JAMA Oncol. 2021 Oct 1;7(10):1578-1579. doi: 10.1001/jamaoncol.2021.3341. JAMA Oncol. 2021. PMID: 34436547 No abstract available.
-
Randomized Clinical Trials in the Era of Precision Oncology-The Role of End Points, Industry Funding, and Medical Writing Integrity.JAMA Oncol. 2021 Oct 1;7(10):1577-1578. doi: 10.1001/jamaoncol.2021.3338. JAMA Oncol. 2021. PMID: 34436567 No abstract available.
-
Randomized Clinical Trials in the Era of Precision Oncology-The Role of End Points, Industry Funding, and Medical Writing Integrity.JAMA Oncol. 2021 Oct 1;7(10):1577. doi: 10.1001/jamaoncol.2021.3335. JAMA Oncol. 2021. PMID: 34436578 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

