Blood Flow Velocimetry in a Microchannel During Coagulation Using Particle Image Velocimetry and Wavelet-Based Optical Flow Velocimetry
- PMID: 33764427
- PMCID: PMC8299809
- DOI: 10.1115/1.4050647
Blood Flow Velocimetry in a Microchannel During Coagulation Using Particle Image Velocimetry and Wavelet-Based Optical Flow Velocimetry
Abstract
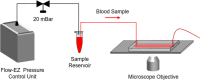
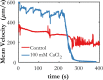
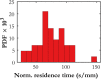
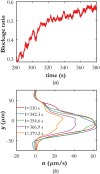
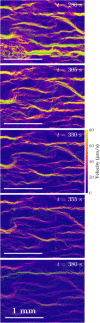
This article describes novel measurements of the velocity of whole blood flow in a microchannel during coagulation. The blood is imaged volumetrically using a simple optical setup involving a white light source and a microscope camera. The images are processed using particle image velocimetry (PIV) and wavelet-based optical flow velocimetry (wOFV), both of which use images of individual blood cells as flow tracers. Measurements of several clinically relevant parameters such as the clotting time, decay rate, and blockage ratio are computed. The high-resolution wOFV results yield highly detailed information regarding thrombus formation and corresponding flow evolution that is the first of its kind.
Copyright © 2021 by ASME.
Figures



Similar articles
-
Assessment and application of wavelet-based optical flow velocimetry (wOFV) to wall-bounded turbulent flows.Exp Fluids. 2023;64(3):50. doi: 10.1007/s00348-023-03594-y. Epub 2023 Feb 21. Exp Fluids. 2023. PMID: 36844890 Free PMC article.
-
Optimized Time-Resolved Echo Particle Image Velocimetry- Particle Tracking Velocimetry Measurements Elucidate Blood Flow in Patients With Left Ventricular Thrombus.J Biomech Eng. 2018 Apr 1;140(4). doi: 10.1115/1.4038886. J Biomech Eng. 2018. PMID: 29305613
-
Smartphone-based particle tracking velocimetry for the in vitro assessment of coronary flows.Med Eng Phys. 2024 Apr;126:104144. doi: 10.1016/j.medengphy.2024.104144. Epub 2024 Mar 11. Med Eng Phys. 2024. PMID: 38621846
-
Measurement of Wall Shear Stress Exerted by Flowing Blood in the Human Carotid Artery: Ultrasound Doppler Velocimetry and Echo Particle Image Velocimetry.Ultrasound Med Biol. 2018 Jul;44(7):1392-1401. doi: 10.1016/j.ultrasmedbio.2018.02.013. Epub 2018 Apr 17. Ultrasound Med Biol. 2018. PMID: 29678322 Free PMC article.
-
On the Quantification of Cellular Velocity Fields.Biophys J. 2016 Apr 12;110(7):1469-1475. doi: 10.1016/j.bpj.2016.02.032. Biophys J. 2016. PMID: 27074673 Free PMC article. Review.
Cited by
-
A microfluidic device for assessment of E-selectin-mediated neutrophil recruitment to inflamed endothelium and prediction of therapeutic response in sickle cell disease.Biosens Bioelectron. 2023 Feb 15;222:114921. doi: 10.1016/j.bios.2022.114921. Epub 2022 Nov 24. Biosens Bioelectron. 2023. PMID: 36521205 Free PMC article.
-
Microfluidics Approach to the Mechanical Properties of Red Blood Cell Membrane and Their Effect on Blood Rheology.Membranes (Basel). 2022 Feb 13;12(2):217. doi: 10.3390/membranes12020217. Membranes (Basel). 2022. PMID: 35207138 Free PMC article.
-
Optical Flow-Based Full-Field Quantitative Blood-Flow Velocimetry Using Temporal Direction Filtering and Peak Interpolation.Int J Mol Sci. 2023 Jul 27;24(15):12048. doi: 10.3390/ijms241512048. Int J Mol Sci. 2023. PMID: 37569421 Free PMC article.
-
Normalization of Blood Viscosity According to the Hematocrit and the Shear Rate.Micromachines (Basel). 2022 Feb 24;13(3):357. doi: 10.3390/mi13030357. Micromachines (Basel). 2022. PMID: 35334649 Free PMC article.
-
Effects of Drag-Reducing Polymers on Hemodynamics and Whole Blood-Endothelial Interactions in 3D-Printed Vascular Topologies.ACS Appl Mater Interfaces. 2024 Mar 27;16(12):14457-14466. doi: 10.1021/acsami.3c17099. Epub 2024 Mar 15. ACS Appl Mater Interfaces. 2024. PMID: 38488736 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

