Access to innovation through the national early access program and clinical trials for patients with malignant melanoma
- PMID: 33764524
- PMCID: PMC8252498
- DOI: 10.1002/cncr.33492
Access to innovation through the national early access program and clinical trials for patients with malignant melanoma
Abstract
Background: The arrival of immunotherapies and targeted therapies challenged the authorities to make them available as soon as possible. France has effective tools, such as clinical trials (CTs) and a national early access program (temporary authorizations for use [ATUs] and temporary recommendations for use [RTUs]), allowing the use of innovative drugs, whether or not they have been authorized or used off-label, for cases that have reached a therapeutic impasse.
Methods: The methodology involved real-time data collection from ATUs, RTUs (between September 1, 2009 and September 1, 2019), and CT authorizations (from December 1, 2017 to September 1, 2019) that were filed and reviewed by the French National Agency for Medicines for metastatic melanoma (MM).
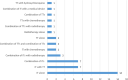
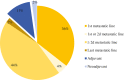
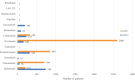
Results: In total, 45 CTs were authorized for MM (51% early phase trials and 44% phase 2 and 3 trials), mainly for the metastatic line (86%) and with an industrial sponsor (73%). Immunotherapies and targeted therapies (63% and 24%, respectively) mostly were used in combination. Three RTUs were authorized for the adjuvant treatment of MM, whereas 13 drugs were available through nominal ATUs (nATUs), of which 5 were awarded a cohort ATU (cATU). This enabled the treatment of 6538 patients (28% through nATUs and 72% through cATUs). All of these drugs were granted marketing authorization and were included in the reimbursement list.
Conclusions: Thanks to CTs and the national early access program, patients in France have been able to benefit from innovative MM treatments.
Lay summary: Several tools allow the use of innovative drugs in France, even if they are not yet authorized or used off-label. From December 1, 2017 to September 1, 2019, 45 clinical trials have been authorized for metastatic melanoma, mostly using immunotherapy (63%) and targeted therapy (24%) at an early phase (51%). Since 2010, the national early access program has treated 6538 patients, including 28% under nominative temporary authorizations for use and 72% under cohort temporary authorizations for use. Fourteen drugs are available through nominative temporary authorizations for use, and 5 are available through cohort temporary authorizations for use, and all of these drugs were granted marketing authorization.
Keywords: France; clinical trials; compassionate use trials; data collection; delivery of health care; drugs investigational; empathy; humans; malignant melanoma.
© 2021 The Authors. Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
The authors made no disclosures.
Figures


Comment in
-
National early access programs and clinical trials: What opportunities for early access to therapeutic innovations for patients with malignant melanoma?Cancer. 2021 Jul 1;127(13):2181-2183. doi: 10.1002/cncr.33495. Epub 2021 Mar 31. Cancer. 2021. PMID: 33788953 No abstract available.
References
-
- Societe Francaise de Dermatologie . Patients Atteints de Melanome de Stade III Inoperable ou de Stade IV—Recommandations et Referentiels. I'Institut National du Cancer (INCa); 2017.
-
- The European Parliament and the Council of the European Union . Directive 2001/20/EC on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Official Journal of the European Communities; 2001. Accessed September 23, 2019. https://eur‐lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2001:121:003... - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

