Mixotrophic growth of the extremophile Galdieria sulphuraria reveals the flexibility of its carbon assimilation metabolism
- PMID: 33764540
- PMCID: PMC8252106
- DOI: 10.1111/nph.17359
Mixotrophic growth of the extremophile Galdieria sulphuraria reveals the flexibility of its carbon assimilation metabolism
Abstract
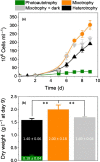
Galdieria sulphuraria is a cosmopolitan microalga found in volcanic hot springs and calderas. It grows at low pH in photoautotrophic (use of light as a source of energy) or heterotrophic (respiration as a source of energy) conditions, using an unusually broad range of organic carbon sources. Previous data suggested that G. sulphuraria cannot grow mixotrophically (simultaneously exploiting light and organic carbon as energy sources), its photosynthetic machinery being repressed by organic carbon. Here, we show that G. sulphuraria SAG21.92 thrives in photoautotrophy, heterotrophy and mixotrophy. By comparing growth, biomass production, photosynthetic and respiratory performances in these three trophic modes, we show that addition of organic carbon to cultures (mixotrophy) relieves inorganic carbon limitation of photosynthesis thanks to increased CO2 supply through respiration. This synergistic effect is lost when inorganic carbon limitation is artificially overcome by saturating photosynthesis with added external CO2 . Proteomic and metabolic profiling corroborates this conclusion suggesting that mixotrophy is an opportunistic mechanism to increase intracellular CO2 concentration under physiological conditions, boosting photosynthesis by enhancing the carboxylation activity of Ribulose-1,5-bisphosphate carboxylase-oxygenase (Rubisco) and decreasing photorespiration. We discuss possible implications of these findings for the ecological success of Galdieria in extreme environments and for biotechnological applications.
Keywords: Galdieria sulphuraria; mixotrophy; photorespiration; photosynthesis; red algae.
© 2021 The Authors New Phytologist © 2021 New Phytologist Foundation.
Figures



References
-
- Allen GJ. 1959. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Archiv für Mikrobiologie 32: 270–277. - PubMed
-
- Avelange MH, Thiéry JM, Sarrey F, Gans P, Rébeillé F. 1988. Mass‐spectrometric determination of O2 and CO2 gas exchange in illuminated higher‐plant cells. Planta 183: 150–157. - PubMed
-
- Azúa‐Bustos A, González‐Silva C, Mancilla RA, Salas L, Palma RE, Wynne JJ, McKay CP, Vicuña R. 2009. Ancient photosynthetic eukaryote biofilms in an Atacama Desert coastal cave. Microbial Ecology 58: 485–496. - PubMed
-
- Barbier G, Oesterhelt C, Larson MD, Halgren RG, Wilkerson C, Garavito RM, Benning C, Weber APM. 2005. Comparative genomics of two closely related unicellular thermo‐acidophilic red algae, Galdieria sulphuraria and Cyanidioschyzon merolae, reveals the molecular basis of the metabolic flexibility of Galdieria sulphuraria and significant differences in carbohydrate metabolism of both algae. Plant Physiology 137: 460–474. - PMC - PubMed
-
- Barcyté D, Nedbalova L, Culka A, Kosek F, Jehlicka J. 2018. Burning coal spoil heaps as a new habitat for the extremophilic red alga Galdieria sulphuraria . Fottea 18: 19–29.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

