An evaluation of the safety and preliminary efficacy of peri- and post-operative treprostinil in preventing ischemia and reperfusion injury in adult orthotopic liver transplant recipients
- PMID: 33764591
- PMCID: PMC8243925
- DOI: 10.1111/ctr.14298
An evaluation of the safety and preliminary efficacy of peri- and post-operative treprostinil in preventing ischemia and reperfusion injury in adult orthotopic liver transplant recipients
Abstract
Background: Orthotopic liver transplantation (OLT) is the only treatment option for various end-stage liver diseases. Ischemia and reperfusion (I/R) injury is one of the unavoidable complications/conditions in OLT. In 2019, a total of 8896 livers were transplanted of which >94% organs were procured from deceased donors. An increase in the use of extended criteria donor (ECD) livers for transplantation further unraveled the role of hepatic I/R injury on short-term and long-term graft outcomes. Despite promising outcomes with the use of antioxidants, free radical scavengers, and vasodilators; I/R-mediated liver injury persists and significantly influences the overall clinical outcomes. Treprostinil, a synthetic prostacyclin I2 (PGI2 ) analog, due to its vasodilatory property, antiplatelet activity, and its ability to downregulate pro-inflammatory cytokines can potentially minimize I/R injury.
Aim: We investigated the safety and preliminary efficacy of continuous intravenous infusion of treprostinil in liver transplant recipients in a prospective, single-center, non-randomized, interventional study.
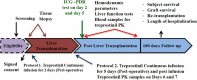
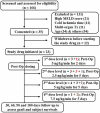
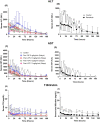
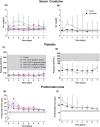
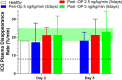
Material and methods: This was a dose escalation (3 + 3 design) phase 1/2 study. Deceased donor liver transplant recipients received 5 ng/kg/min for two days, or 2.5, 5, and 7.5 ng/min/kg for 5 days as a continuous infusion. Multiple blood samples were collected for biochemical parameter assessment and for measuring treprostinil levels. Indocyanine green plasma disappearance rate was used as a measure of hepatic functional capacity.
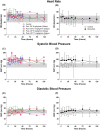
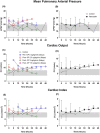
Results: Subjects tolerated continuous infusion of treprostinil up to 5 ng/kg/min for 120 h with no occurrence of primary graft non-function (PNF), minimized need for ventilation support, reduced hospitalization time, 100% graft and patient survival, and improved hepatobiliary excretory function comparable to normal healthy adults.
Discussion: Treprostinil can be administered to liver transplant patients safely during the perioperative period.
Conclusion: Based on this phase 1/2 study, further efficacy studies of treprostinil in preventing I/R injury of liver should be conducted to potentially increase the number of livers available for transplantation.
Keywords: ischemia reperfusion injury (IRI); liver transplantation; treprostinil.
© 2021 The Authors. Clinical Transplantation published by John Wiley & Sons Ltd.
Figures

References
-
- Ascher NL. Liver transplantation. In: Townsend CMB, Evers RD, Mattox KL, eds. Sabiston Textbook Of Surgery (19th edn). Philadelphia, PA: Elsevier In. 2012:655‐665.
-
- (OPTN) OPaTN . Liver Organ Stats, Scientific Registry of Transplant Recipients. Richmond, VA: The U.S. Department of Health and Human Services. 2016.
-
- Kim WR, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 annual data report: liver. Am J Transplant. 2014;14(S1):69‐96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

