Effects of dosing frequency on the clinical efficacy of ampicillin/sulbactam in Japanese elderly patients with pneumonia: A single-center retrospective observational study
- PMID: 33764686
- PMCID: PMC7992287
- DOI: 10.1002/prp2.746
Effects of dosing frequency on the clinical efficacy of ampicillin/sulbactam in Japanese elderly patients with pneumonia: A single-center retrospective observational study
Abstract
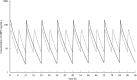
This study sought to investigate whether dosing frequency (the number of doses per day) affects the antimicrobial efficacy and safety of ampicillin/sulbactam (ABPC/SBT) in Japanese elderly pneumonia patients treated with ABPC/SBT at 6 g/day. This was a retrospective observational study that included hospitalized elderly patients (aged ≥75 years, 10 ml/min ≤CLcr <50 ml/min) who received 3 g every 12 h (BID; n = 61) or 1.5 g every 6 h (QID; n = 45) for the treatment of pneumonia. The primary endpoint was clinical response, assessed by measuring body temperature, white blood cell count, and C-reactive protein levels. Pharmacokinetic and pharmacodynamic simulations were conducted in silico to rationalize the clinical findings. The clinical response rates (extremely effective and effective) in the BID and QID groups were 36.1% and 55.6%, respectively (p = .0459). QID tended to be more effective in patients with gram-negative rods detected (p = .0563). According to the simulated minimum plasma ABPC concentrations at steady state for BID and QID were 2.5 and 7.3 μg/ml, respectively (p < .0001). Based on the simulated time above minimum inhibitory concentration (MIC), pharmacological (not clinical) efficacy was predicted to be higher with QID. Both groups had similar safety profiles. The main adverse event in both groups was liver damage. The present retrospective survey demonstrated that ABPC/SBT treatment for elderly patients with pneumonia and renal dysfunction was more effective with QID than with BID. Therefore, the QID regimen is worthy of consideration to improve the clinical outcomes of ABPC/SBT therapy in the present patient population.
Keywords: ampicillin/sulbactam; elderly patients; pharmacokinetics-pharmacodynamics; pneumonia; retrospective study.
© 2021 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- The JRS Guidelines for the Management of Pneumonia in Adults 2017. Medical Review. 2017;xix;175p.
-
- USP UNASYN . https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050608s044lbl.pdf. Accessed November 17, 2019.
-
- Kohno S, Tateda K, Mikamo H, Kadota J, Niki Y, Itamura R. Efficacy and safety of intravenous sulbactam/ampicillin 3 g 4 times daily in Japanese adults with moderate to severe community‐acquired pneumonia: a multicenter, open‐label, uncontrolled study. J Infect Chemother. 2015;21:182‐188. 10.1016/j.jiac.2014.11.006 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

