DMV extrasynaptic NMDA receptors regulate caloric intake in rats
- PMID: 33764905
- PMCID: PMC8262316
- DOI: 10.1172/jci.insight.139785
DMV extrasynaptic NMDA receptors regulate caloric intake in rats
Abstract
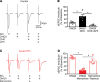
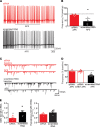
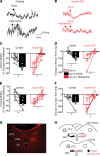
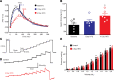
Acute high-fat diet (aHFD) exposure induces a brief period of hyperphagia before caloric balance is restored. Previous studies have demonstrated that this period of regulation is associated with activation of synaptic N-methyl-D-aspartate (NMDA) receptors on dorsal motor nucleus of the vagus (DMV) neurons, which increases vagal control of gastric functions. Our aim was to test the hypothesis that activation of DMV synaptic NMDA receptors occurs subsequent to activation of extrasynaptic NMDA receptors. Sprague-Dawley rats were fed a control or high-fat diet for 3-5 days prior to experimentation. Whole-cell patch-clamp recordings from gastric-projecting DMV neurons; in vivo recordings of gastric motility, tone, compliance, and emptying; and food intake studies were used to assess the effects of NMDA receptor antagonism on caloric regulation. After aHFD exposure, inhibition of extrasynaptic NMDA receptors prevented the synaptic NMDA receptor-mediated increase in glutamatergic transmission to DMV neurons, as well as the increase in gastric tone and motility, while chronic extrasynaptic NMDA receptor inhibition attenuated the regulation of caloric intake. After aHFD exposure, the regulation of food intake involved synaptic NMDA receptor-mediated currents, which occurred in response to extrasynaptic NMDA receptor activation. Understanding these events may provide a mechanistic basis for hyperphagia and may identify novel therapeutic targets for the treatment of obesity.
Keywords: Gastroenterology; Ion channels; Neuroscience; Obesity; Synapses.
Conflict of interest statement
Figures


Similar articles
-
Brainstem astrocytes control homeostatic regulation of caloric intake.J Physiol. 2023 Feb;601(4):801-829. doi: 10.1113/JP283566. Epub 2023 Jan 25. J Physiol. 2023. PMID: 36696965 Free PMC article.
-
Acute high-fat diet upregulates glutamatergic signaling in the dorsal motor nucleus of the vagus.Am J Physiol Gastrointest Liver Physiol. 2018 May 1;314(5):G623-G634. doi: 10.1152/ajpgi.00395.2017. Epub 2018 Jan 25. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29368945 Free PMC article.
-
Perinatal high-fat diet alters development of GABAA receptor subunits in dorsal motor nucleus of vagus.Am J Physiol Gastrointest Liver Physiol. 2019 Jul 1;317(1):G40-G50. doi: 10.1152/ajpgi.00079.2019. Epub 2019 May 1. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31042399 Free PMC article.
-
A tale of two endings: modulation of satiation by NMDA receptors on or near central and peripheral vagal afferent terminals.Physiol Behav. 2011 Nov 30;105(1):94-9. doi: 10.1016/j.physbeh.2011.02.042. Epub 2011 Mar 5. Physiol Behav. 2011. PMID: 21382391 Free PMC article. Review.
-
Glutamatergic plasticity within neurocircuits of the dorsal vagal complex and the regulation of gastric functions.Am J Physiol Gastrointest Liver Physiol. 2021 May 1;320(5):G880-G887. doi: 10.1152/ajpgi.00014.2021. Epub 2021 Mar 17. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33730858 Free PMC article. Review.
Cited by
-
Brain-gut communication: vagovagal reflexes interconnect the two "brains".Am J Physiol Gastrointest Liver Physiol. 2021 Nov 1;321(5):G576-G587. doi: 10.1152/ajpgi.00214.2021. Epub 2021 Oct 13. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 34643086 Free PMC article. Review.
-
Perinatal high-fat diet exposure alters oxytocin and corticotropin releasing factor inputs onto vagal neurocircuits controlling gastric motility.J Physiol. 2023 Jul;601(14):2853-2875. doi: 10.1113/JP284726. Epub 2023 May 17. J Physiol. 2023. PMID: 37154244 Free PMC article.
-
Misalignment of Circadian Rhythms in Diet-Induced Obesity.Adv Exp Med Biol. 2024;1460:27-71. doi: 10.1007/978-3-031-63657-8_2. Adv Exp Med Biol. 2024. PMID: 39287848 Review.
-
Glial Modulation of Energy Balance: The Dorsal Vagal Complex Is No Exception.Int J Mol Sci. 2022 Jan 16;23(2):960. doi: 10.3390/ijms23020960. Int J Mol Sci. 2022. PMID: 35055143 Free PMC article. Review.
-
Brainstem astrocytes control homeostatic regulation of caloric intake.J Physiol. 2023 Feb;601(4):801-829. doi: 10.1113/JP283566. Epub 2023 Jan 25. J Physiol. 2023. PMID: 36696965 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

