Identifying optimal COVID-19 testing strategies for schools and businesses: Balancing testing frequency, individual test technology, and cost
- PMID: 33764982
- PMCID: PMC7993807
- DOI: 10.1371/journal.pone.0248783
Identifying optimal COVID-19 testing strategies for schools and businesses: Balancing testing frequency, individual test technology, and cost
Abstract
Background: COVID-19 test sensitivity and specificity have been widely examined and discussed, yet optimal use of these tests will depend on the goals of testing, the population or setting, and the anticipated underlying disease prevalence. We model various combinations of key variables to identify and compare a range of effective and practical surveillance strategies for schools and businesses.
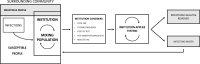
Methods: We coupled a simulated data set incorporating actual community prevalence and test performance characteristics to a susceptible, infectious, removed (SIR) compartmental model, modeling the impact of base and tunable variables including test sensitivity, testing frequency, results lag, sample pooling, disease prevalence, externally-acquired infections, symptom checking, and test cost on outcomes including case reduction and false positives.
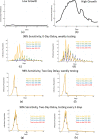
Findings: Increasing testing frequency was associated with a non-linear positive effect on cases averted over 100 days. While precise reductions in cumulative number of infections depended on community disease prevalence, testing every 3 days versus every 14 days (even with a lower sensitivity test) reduces the disease burden substantially. Pooling provided cost savings and made a high-frequency approach practical; one high-performing strategy, testing every 3 days, yielded per person per day costs as low as $1.32.
Interpretation: A range of practically viable testing strategies emerged for schools and businesses. Key characteristics of these strategies include high frequency testing with a moderate or high sensitivity test and minimal results delay. Sample pooling allowed for operational efficiency and cost savings with minimal loss of model performance.
Conflict of interest statement
Authors [GL, NS, CK, DG, EB] are employees of UnitedHealth Group; GL, NS, CK, and EB own stock in the company. DG is employed as the Senior Infectious Disease Fellow at UnitedHealth Group, Inc and serves as the Chief of Infectious Diseases for ProHealth NY an Optum Company. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Service RF. Coronavirus antigen tests: quick and cheap, but too often wrong? Science | AAAS. 2020. Available from: https://www.sciencemag.org/news/2020/05/coronavirus-antigen-tests-quick-...
-
- FDA. Emergency Use Authorization. FDA. 2020. Available from: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regula...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

