Immune mediation of HMG-like DSP1 via Toll-Spätzle pathway and its specific inhibition by salicylic acid analogs
- PMID: 33765093
- PMCID: PMC8023496
- DOI: 10.1371/journal.ppat.1009467
Immune mediation of HMG-like DSP1 via Toll-Spätzle pathway and its specific inhibition by salicylic acid analogs
Abstract
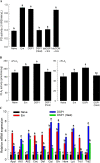
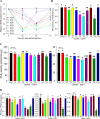
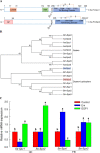
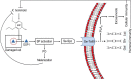
Xenorhabdus hominickii, an entomopathogenic bacterium, inhibits eicosanoid biosynthesis of target insects to suppress their immune responses by inhibiting phospholipase A2 (PLA2) through binding to a damage-associated molecular pattern (DAMP) molecule called dorsal switch protein 1 (DSP1) from Spodoptera exigua, a lepidopteran insect. However, the signalling pathway between DSP1 and PLA2 remains unknown. The objective of this study was to determine whether DSP1 could activate Toll immune signalling pathway to activate PLA2 activation and whether X. hominickii metabolites could inhibit DSP1 to shutdown eicosanoid biosynthesis. Toll-Spätzle (Spz) signalling pathway includes two Spz (SeSpz1 and SeSpz2) and 10 Toll receptors (SeToll1-10) in S. exigua. Loss-of-function approach using RNA interference showed that SeSpz1 and SeToll9 played crucial roles in connecting DSP1 mediation to activate PLA2. Furthermore, a deletion mutant against SeToll9 using CRISPR/Cas9 abolished DSP1 mediation and induced significant immunosuppression. Organic extracts of X. hominickii culture broth could bind to DSP1 at a low micromolar range. Subsequent sequential fractionations along with binding assays led to the identification of seven potent compounds including 3-ethoxy-4-methoxyphenol (EMP). EMP could bind to DSP1 and prevent its translocation to plasma in response to bacterial challenge and suppress the up-regulation of PLA2 activity. These results suggest that X. hominickii inhibits DSP1 and prevents its DAMP role in activating Toll immune signalling pathway including PLA2 activation, leading to significant immunosuppression of target insects.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures









Similar articles
-
Specific inhibition of Xenorhabdus hominickii, an entomopathogenic bacterium, against different types of host insect phospholipase A2.J Invertebr Pathol. 2017 Oct;149:97-105. doi: 10.1016/j.jip.2017.08.009. Epub 2017 Aug 10. J Invertebr Pathol. 2017. PMID: 28803982
-
Spätzle processing enzyme is required to activate dorsal switch protein 1 induced Toll immune signalling pathway in Tenebrio molitor.PLoS One. 2023 Sep 21;18(9):e0291976. doi: 10.1371/journal.pone.0291976. eCollection 2023. PLoS One. 2023. PMID: 37733725 Free PMC article.
-
Damage signal induced by Bacillus thuringiensis infection triggers immune responses via a DAMP molecule in lepidopteran insect, Spodoptera exigua.Dev Comp Immunol. 2023 Feb;139:104559. doi: 10.1016/j.dci.2022.104559. Epub 2022 Sep 29. Dev Comp Immunol. 2023. PMID: 36181778
-
Eicosanoid-mediated immunity in insects.Dev Comp Immunol. 2018 Jun;83:130-143. doi: 10.1016/j.dci.2017.12.005. Epub 2017 Dec 7. Dev Comp Immunol. 2018. PMID: 29225005 Review.
-
Dopamine-1 receptor G-protein coupling and the involvement of phospholipase A2 in dopamine-1 receptor mediated cellular signaling mechanisms in the proximal tubules of SHR.Clin Exp Hypertens. 1997 Jan-Feb;19(1-2):131-40. doi: 10.3109/10641969709080810. Clin Exp Hypertens. 1997. PMID: 9028641 Review.
Cited by
-
Dorsal switch protein 1 as a damage signal in insect gut immunity to activate dual oxidase via an eicosanoid, PGE2.Front Immunol. 2022 Nov 10;13:994626. doi: 10.3389/fimmu.2022.994626. eCollection 2022. Front Immunol. 2022. PMID: 36439105 Free PMC article.
-
Emerging Evidence on Tenebrio molitor Immunity: A Focus on Gene Expression Involved in Microbial Infection for Host-Pathogen Interaction Studies.Microorganisms. 2022 Oct 7;10(10):1983. doi: 10.3390/microorganisms10101983. Microorganisms. 2022. PMID: 36296259 Free PMC article. Review.
-
Salicylic Acid, a Plant Hormone, Suppresses Phytophagous Insect Immune Response by Interrupting HMG-Like DSP1.Front Physiol. 2021 Oct 4;12:744272. doi: 10.3389/fphys.2021.744272. eCollection 2021. Front Physiol. 2021. PMID: 34671276 Free PMC article.
-
HMGB1-Like Dorsal Switch Protein 1 Triggers a Damage Signal in Mosquito Gut to Activate Dual Oxidase via Eicosanoids.J Innate Immun. 2022;14(6):657-672. doi: 10.1159/000524561. Epub 2022 May 5. J Innate Immun. 2022. PMID: 35512659 Free PMC article.
-
Ligands of HMG-like dorsal switch protein 1 of Spodoptera exigua leads to mortality in diamondback moth, Plutellaxylostella.Heliyon. 2024 Mar 9;10(6):e27090. doi: 10.1016/j.heliyon.2024.e27090. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38509914 Free PMC article.
References
-
- Snyder H, Stock SP, Kim SK, Flores-Lara Y, Forst S. New insights into the colonization and release processes of Xenorhabdus nematophila and the morphology and ultrastructure of the bacterial receptacle of its nematode host, Steinernema carpocapsae. Appl Environ Microbiol. 2007; 73: 5338–5346. 10.1128/AEM.02947-06 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

