Distinct bone marrow immunophenotypic features define the splicing factor 3B subunit 1 (SF3B1)-mutant myelodysplastic syndromes subtype
- PMID: 33765355
- PMCID: PMC8252736
- DOI: 10.1111/bjh.17414
Distinct bone marrow immunophenotypic features define the splicing factor 3B subunit 1 (SF3B1)-mutant myelodysplastic syndromes subtype
Abstract
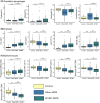
Splicing factor 3B subunit 1 (SF3B1) mutations define a distinct myelodysplastic syndromes (MDS) patient group with a relatively favourable disease course and high response rates to luspatercept. Few data are available on bone marrow phenotype beyond ring sideroblasts in this subgroup of patients with MDS. In the present study, we identified immunophenotypic erythroid, myelomonocyte and progenitor features associated with SF3B1 mutations. In addition, we illustrate that SF3B1-mutation type is associated with distinct immunophenotypic features, and show the impact of co-occurrence of a SF3B1 mutation and a deletion of chromosome 5q on bone marrow immunophenotype. These genotype-phenotype associations and phenotypic subtypes within SF3B1-MDS provide leads that may further refine prognostication and therapeutic strategies for this particular MDS subgroup.
Keywords: SF3B1; diagnostic haematology; flow cytometry; mutational analysis; myelodysplastic syndromes.
© 2021 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Fenaux P, Platzbecker U, Mufti GJ, Garcia‐Manero G, Buckstein R, Santini V, et al. Luspatercept in patients with lower‐risk myelodysplastic syndromes. N Engl J Med. 2020;382:140–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

