Successful and Unsuccessful Prediction of Human Hepatic Clearance for Lead Optimization
- PMID: 33765384
- PMCID: PMC8504179
- DOI: 10.1021/acs.jmedchem.0c01930
Successful and Unsuccessful Prediction of Human Hepatic Clearance for Lead Optimization
Abstract
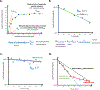
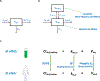
Development of new chemical entities is costly, time-consuming, and has a low success rate. Accurate prediction of pharmacokinetic properties is critical to progress compounds with favorable drug-like characteristics in lead optimization. Of particular importance is the prediction of hepatic clearance, which determines drug exposure and contributes to projection of dose, half-life, and bioavailability. The most commonly employed methodology to predict hepatic clearance is termed in vitro to in vivo extrapolation (IVIVE) that involves measuring drug metabolism in vitro, scaling-up this in vitro intrinsic clearance to a prediction of in vivo intrinsic clearance by reconciling the enzymatic content between the incubation and an average human liver, and applying a model of hepatic disposition to account for limitations of protein binding and blood flow to predict in vivo clearance. This manuscript reviews common in vitro techniques used to predict hepatic clearance as well as current challenges and recent theoretical advancements in IVIVE.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- DiMasi JA; Grabowski HG; Hansen RW Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ 2016, 47, 20–33. - PubMed
-
- Obach RS; Baxter JG; Liston TE; Silber BM; Jones BC; MacIntyre F; Rance DJ; Wastall P The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J. Pharmacol. Exp. Ther 1997, 283, 46–58. - PubMed
-
- Rowland M; Benet LZ; Graham GG Clearance concepts in pharmacokinetics. J. Pharmacokinet. Biopharm 1973, 1, 123–135. - PubMed
-
- Wilkinson GR; Shand DG Commentary: a physiologic approach to hepatic drug clearance. Clin. Pharmacol. Ther 1975, 18, 377–390. - PubMed
-
- Strom SC; Jirtle RL; Jones RS; Novicki DL; Rosenberg MR; Novonty A; Irons G; McLain JR; Michalopoulos G Isolation, culture, and transplantation of human hepatocytes. J. Natl. Cancer Inst 1982, 68 (50), 771–778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

