An early cell shape transition drives evolutionary expansion of the human forebrain
- PMID: 33765444
- PMCID: PMC8054913
- DOI: 10.1016/j.cell.2021.02.050
An early cell shape transition drives evolutionary expansion of the human forebrain
Abstract
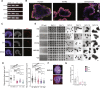
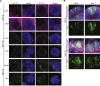
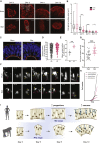
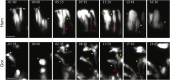
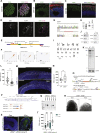
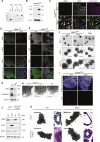
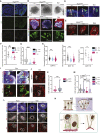
The human brain has undergone rapid expansion since humans diverged from other great apes, but the mechanism of this human-specific enlargement is still unknown. Here, we use cerebral organoids derived from human, gorilla, and chimpanzee cells to study developmental mechanisms driving evolutionary brain expansion. We find that neuroepithelial differentiation is a protracted process in apes, involving a previously unrecognized transition state characterized by a change in cell shape. Furthermore, we show that human organoids are larger due to a delay in this transition, associated with differences in interkinetic nuclear migration and cell cycle length. Comparative RNA sequencing (RNA-seq) reveals differences in expression dynamics of cell morphogenesis factors, including ZEB2, a known epithelial-mesenchymal transition regulator. We show that ZEB2 promotes neuroepithelial transition, and its manipulation and downstream signaling leads to acquisition of nonhuman ape architecture in the human context and vice versa, establishing an important role for neuroepithelial cell shape in human brain expansion.
Keywords: ZEB2; brain; brain expansion; cell shape; chimpanzee; evolution; gorilla; neural stem cells; neuroepithelium; organoids.
Copyright © 2021 MRC Laboratory of Molecular Biology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures








Comment in
-
Shaping the human brain.Nat Rev Mol Cell Biol. 2021 May;22(5):304-305. doi: 10.1038/s41580-021-00364-8. Nat Rev Mol Cell Biol. 2021. PMID: 33782586 No abstract available.
-
Founder cells shape brain evolution.Cell. 2021 Apr 15;184(8):1965-1967. doi: 10.1016/j.cell.2021.03.045. Cell. 2021. PMID: 33861961
References
-
- Aaku-Saraste E., Hellwig A., Huttner W.B. Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure--remodeling of the neuroepithelium prior to neurogenesis. Dev. Biol. 1996;180:664–679. - PubMed
-
- Amat F., Höckendorf B., Wan Y., Lemon W.C., McDole K., Keller P.J. Efficient processing and analysis of large-scale light-sheet microscopy data. Nat. Protoc. 2015;10:1679–1696. - PubMed
-
- Ando-Akatsuka Y., Yonemura S., Itoh M., Furuse M., Tsukita S. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J. Cell. Physiol. 1999;179:115–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

