A pattern-triggered immunity-related phenolic, acetosyringone, boosts rapid inhibition of a diverse set of plant pathogenic bacteria
- PMID: 33765920
- PMCID: PMC7992983
- DOI: 10.1186/s12870-021-02928-4
A pattern-triggered immunity-related phenolic, acetosyringone, boosts rapid inhibition of a diverse set of plant pathogenic bacteria
Abstract
Background: Acetosyringone (3,5-dimethoxy-4-hydroxyacetophenone, AS) is a syringyl-type phenolic compound rarely found in plants in free form. It has been shown earlier to inhibit the growth of Pseudomonas bacteria in the presence of hydrogen peroxide and peroxidase (AS mix).
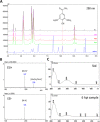
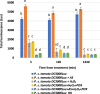
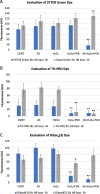
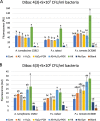
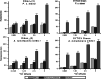
Results: We detected elevated levels of free AS in Nicotiana tabacum and N. benthamiana plants after inducing pattern-triggered immunity (PTI) by injecting bacterial elicitor flg22, or pathogenicity-mutant Pseudomonas syringae pv. syringae 61 hrcC- bacteria; but not after inoculations with compatible or incompatible pathogens at the time of PTI onset. In this study, we demonstrate that the antibacterial effect of the AS mix is general, as growth of several Gram-negative and -positive phytopathogenic bacteria was characteristically inhibited. The inhibition of bacterial metabolism by the AS mix was rapid, shown by the immediate drop of luminescence intensity of P. syringae pv. tomato DC3000 lx strain after addition of AS mix. The mechanism of the bacteriostatic effect was investigated using fluorescent reporter dye assays. SYTOX Green experiments supported others' previous findings that the AS mix does not result in membrane permeabilization. Moreover, we observed that the mode of action could be depolarization of the bacterial cell membrane, as shown by assays carried out with the voltage sensitive dye DIBAC4(3).
Conclusions: Level of free acetosyringone is elevated during plant PTI responses in tobacco leaves (N. tabacum and N. benthamiana). When combined with hydrogen peroxide and peroxidase (AS mix), components of the mix act synergistically to inhibit bacterial metabolism and proliferation rapidly in a wide range of plant pathogens. This effect is related to depolarization rather than to permeabilization of the bacterial cell membrane. Similar AS mixture to the in vivo model might form locally at sites of invading bacterial attachment to the plant cells and the presence of acetosyringone might have an important role in the inhibition of bacterial proliferation during PTI.
Keywords: Acetosyringone; Antibacterial; Elicitor; Oxidative burst; Pattern-triggered immunity; Pseudomonas syringae.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- T. Boller, G. Felix, A renaissance of rlicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors, Annu Rev Plant Biol 60 (2009) 379–406. https://doi.org/10.1146/annurev.arplant.57.032905.105346, 1. - PubMed
-
- M.A. Newman, T. Sundelin, J.T. Nielsen, G. Erbs, MAMP (microbe-associated molecular pattern) triggered immunity in plants, Front Plant Sci 4 (2013) 139. https://doi.org/10.3389/fpls.2013.00139. - PMC - PubMed
-
- Z. Bozso, P.G. Ott, A. Szatmari, A. Czelleng, G. Varga, E. Besenyei, E. Sardi, E. Banyai, Z. Klement, Early detection of bacterium-induced basal resistance in tobacco leaves with diaminobenzidine and dichlorofluorescein diacetate, J Phytopathol 153 (2005) 596–607. https://doi.org/10.1111/j.1439-0434.2005.01026.x, 10.
-
- A. Szatmari, P.G. Ott, G.J. Varga, E. Besenyei, A. Czelleng, Z. Klement, Z. Bozsó, Characterisation of basal resistance (BR) by expression patterns of newly isolated representative genes in tobacco, Plant Cell Rep. 25 (2006). https://doi.org/10.1007/s00299-005-0110-5. - PubMed
-
- A.R. Ramos, J.E. Morello, S. Ravindran, W.L. Deng, H.C. Huang, A. Collmer, Identification of Pseudomonas syringae pv. syringae 61 type III secretion system Hrp proteins that can travel the type III pathway and contribute to the translocation of effector proteins into plant cells, J. Bacteriol. 189 (2007) 5773–5778. https://doi.org/10.1128/JB.00435-07. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

