Chlorhexidine for facility-based umbilical cord care: EN-BIRTH multi-country validation study
- PMID: 33765947
- PMCID: PMC7995704
- DOI: 10.1186/s12884-020-03338-4
Chlorhexidine for facility-based umbilical cord care: EN-BIRTH multi-country validation study
Abstract
Background: Umbilical cord hygiene prevents sepsis, a leading cause of neonatal mortality. The World Health Organization recommends 7.1% chlorhexidine digluconate (CHX) application to the umbilicus after home birth in high mortality contexts. In Bangladesh and Nepal, national policies recommend CHX use for all facility births. Population-based household surveys include optional questions on CHX use, but indicator validation studies are lacking. The Every Newborn Birth Indicators Research Tracking in Hospitals (EN-BIRTH) was an observational study assessing measurement validity for maternal and newborn indicators. This paper reports results regarding CHX.
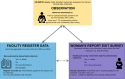
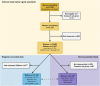
Methods: The EN-BIRTH study (July 2017-July 2018) included three public hospitals in Bangladesh and Nepal where CHX cord application is routine. Clinical-observers collected tablet-based, time-stamped data regarding cord care during admission to labour and delivery wards as the gold standard to assess accuracy of women's report at exit survey, and of routine-register data. We calculated validity ratios and individual-level validation metrics; analysed coverage, quality and measurement gaps. We conducted qualitative interviews to assess barriers and enablers to routine register-recording.
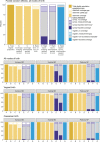
Results: Umbilical cord care was observed for 12,379 live births. Observer-assessed CHX coverage was very high at 89.3-99.4% in all 3 hospitals, although slightly lower after caesarean births in Azimpur (86.8%), Bangladesh. Exit survey-reported coverage (0.4-45.9%) underestimated the observed coverage with substantial "don't know" responses (55.5-79.4%). Survey-reported validity ratios were all poor (0.01 to 0.38). Register-recorded coverage in the specific column in Bangladesh was underestimated by 0.2% in Kushtia but overestimated by 9.0% in Azimpur. Register-recorded validity ratios were good (0.9 to 1.1) in Bangladesh, and poor (0.8) in Nepal. The non-specific register column in Pokhara, Nepal substantially underestimated coverage (20.7%).
Conclusions: Exit survey-report highly underestimated observed CHX coverage in all three hospitals. Routine register-recorded coverage was closer to observer-assessed coverage than survey reports in all hospitals, including for caesarean births, and was more accurately captured in hospitals with a specific register column. Inclusion of CHX cord care into registers, and tallied into health management information system platforms, is justified in countries with national policies for facility-based use, but requires implementation research to assess register design and data flow within health information systems.
Keywords: 7.1% chlorhexidine; Birth; Coverage; Health management systems; Hospital records; Neonatal sepsis; Newborn; Survey; Umbilical cord care; Validity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Blencowe H, Cousens S. Addressing the challenge of neonatal mortality. Tropical Med Int Health. 2013;18(3):303–312. - PubMed
-
- UNICEF . The state of the World’s children 2019. Children, food and nutrition: growing well in a changing world. New York: UNICEF; 2019.
-
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

