Uterotonics for prevention of postpartum haemorrhage: EN-BIRTH multi-country validation study
- PMID: 33765962
- PMCID: PMC7995712
- DOI: 10.1186/s12884-020-03420-x
Uterotonics for prevention of postpartum haemorrhage: EN-BIRTH multi-country validation study
Abstract
Background: Postpartum haemorrhage (PPH) is a leading cause of preventable maternal mortality worldwide. The World Health Organization (WHO) recommends uterotonic administration for every woman after birth to prevent PPH. There are no standardised data collected in large-scale measurement platforms. The Every Newborn Birth Indicators Research Tracking in Hospitals (EN-BIRTH) is an observational study to assess the validity of measurement of maternal and newborn indicators, and this paper reports findings regarding measurement of coverage and quality for uterotonics.
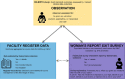
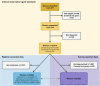
Methods: The EN-BIRTH study took place in five hospitals in Bangladesh, Nepal and Tanzania, from July 2017 to July 2018. Clinical observers collected tablet-based, time-stamped data. We compared observation data for uterotonics to routine hospital register-records and women's report at exit-interview survey. We analysed the coverage and quality gap for timing and dose of administration. The register design was evaluated against gap analyses and qualitative interview data assessing the barriers and enablers to data recording and use.
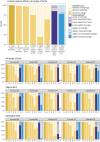
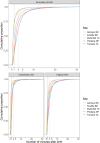
Results: Observed uterotonic coverage was high in all five hospitals (> 99%, 95% CI 98.7-99.8%). Survey-report underestimated coverage (79.5 to 91.7%). "Don't know" replies varied (2.1 to 14.4%) and were higher after caesarean (3.7 to 59.3%). Overall, there was low accuracy in survey data for details of uterotonic administration (type and timing). Register-recorded coverage varied in four hospitals capturing uterotonics in a specific column (21.6, 64.5, 97.6, 99.4%). The average coverage measurement gap was 18.1% for register-recorded and 6.0% for survey-reported coverage. Uterotonics were given to 15.9% of women within the "right time" (1 min) and 69.8% within 3 min. Women's report of knowing the purpose of uterotonics after birth ranged from 0.4 to 64.9% between hospitals. Enabling register design and adequate staffing were reported to improve routine recording.
Conclusions: Routine registers have potential to track uterotonic coverage - register data were highly accurate in two EN-BIRTH hospitals, compared to consistently underestimated coverage by survey-report. Although uterotonic coverage was high, there were gaps in observed quality for timing and dose. Standardisation of register design and implementation could improve data quality and data flow from registers into health management information reporting systems, and requires further assessment.
Keywords: Birth; Coverage; Health management systems; Hospital records; Maternal; Postpartum haemorrhage; Survey; Uterotonics; Validity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- WHO, UNICEF, UNFPA, World Bank Group, United Nations population division . trends in maternal mortality: 2000 to 2017. Geneva: World Health Organization; 2019.
-
- Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, Costa MJ, Fawole B, Mugerwa Y, Nafiou I. Moving beyond essential interventions for reduction of maternal mortality (the WHO multicountry survey on maternal and newborn health): a cross-sectional study. Lancet. 2013;381(9879):1747–1755. doi: 10.1016/S0140-6736(13)60686-8. - DOI - PubMed
-
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health. 2014;2(6):e323–33.. - PubMed
-
- World Health Organization. WHO recommendations Uterotonics for the prevention of postpartum haemorrhage. Geneva: World Health Organization;.. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

