Considerations for applying bioethics norms to a biopharmaceutical industry setting
- PMID: 33766013
- PMCID: PMC7992125
- DOI: 10.1186/s12910-021-00600-y
Considerations for applying bioethics norms to a biopharmaceutical industry setting
Abstract
Background: The biopharmaceutical industry operates at the intersection of life sciences, clinical research, clinical care, public health, and business, which presents distinct operational and ethical challenges. This setting merits focused bioethics consideration to complement legal compliance and business ethics efforts. However, bioethics as applied to a biopharmaceutical industry setting often is construed either too broadly or too narrowly with little examination of its proper scope.
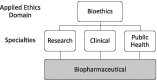
Main text: Any institution with a scientific or healthcare mission should engage bioethics norms to navigate ethical issues that arise from the conduct of biomedical research, delivery of clinical care, or implementation of public health programs. It is reasonable to assume that while bioethics norms must remain constant, their application will vary depending on the characteristics of a given setting. Context "specification" substantively refines ethics norms for a particular discipline or setting and is an expected, needed and progressive ethical activity. In order for this activity to be meaningful, the scope for bioethics application and the relevant contextual factors of the setting need to be delineated and appreciated. This paper defines biopharmaceutical bioethics as: the application of bioethics norms (concepts, principles, and rules) to the research, development, supply, commercialization, and clinical use of biopharmaceutical healthcare products. It provides commentary on this definition, and presents five contextual factors that need to be considered when applying bioethics norms to a biopharmaceutical industry setting: (1) dual missions; (2) timely and pragmatic guidance; (3) resource stewardship; (4) multiple stakeholders; and (5) operational complexity.
Conclusion: Understanding the scope of the biopharmaceutical enterprise and contextual factors of a biopharmaceutical industry setting is foundational for the application of bioethics norms. Establishing a common language and approach for biopharmaceutical bioethics will facilitate breadth and depth of discussion and subsequent implementation to benefit patients, the healthcare system and society.
Keywords: Bioethics norms; Biopharmaceutical industry; Context; Definition; Setting; Specification.
Conflict of interest statement
All authors and members of the Biopharmaceutical Bioethics Working Group have been or are employees and equity holders of biopharmaceutical companies. LVC, DGT, MAT, and some members of the working group have been or are consultants for biopharmaceutical companies. The views expressed in this paper are those of the authors and working group members and do not represent their respective employers, past or present.
Figures

References
-
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). 2020. https://www.ich.org. Accessed 30 July 2020.
-
- Levine RJ. Ethics and regulation of clinical research. 2. New Haven: Yale University Press; 1988.
-
- Beauchamp TL, Childress JF. Principles of biomedical ethics. 6. New York: Oxford University Press; 2009.
-
- The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research . The Belmont report: ethical principles and guidelines for the protection of human subjects of research. Bethesda: The Commission; 1979. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

