H3K27 modifiers regulate lifespan in C. elegans in a context-dependent manner
- PMID: 33766022
- PMCID: PMC7995591
- DOI: 10.1186/s12915-021-00984-8
H3K27 modifiers regulate lifespan in C. elegans in a context-dependent manner
Abstract
Background: Evidence of global heterochromatin decay and aberrant gene expression in models of physiological and premature ageing have long supported the "heterochromatin loss theory of ageing", which proposes that ageing is aetiologically linked to, and accompanied by, a progressive, generalised loss of repressive epigenetic signatures. However, the remarkable plasticity of chromatin conformation suggests that the re-establishment of such marks could potentially revert the transcriptomic architecture of animal cells to a "younger" state, promoting longevity and healthspan. To expand our understanding of the ageing process and its connection to chromatin biology, we screened an RNAi library of chromatin-associated factors for increased longevity phenotypes.
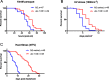
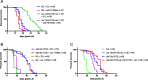
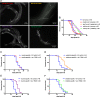
Results: We identified the lysine demethylases jmjd-3.2 and utx-1, as well as the lysine methyltransferase mes-2 as regulators of both lifespan and healthspan in C. elegans. Strikingly, we found that both overexpression and loss of function of jmjd-3.2 and utx-1 are all associated with enhanced longevity. Furthermore, we showed that the catalytic activity of UTX-1, but not JMJD-3.2, is critical for lifespan extension in the context of overexpression. In attempting to reconcile the improved longevity associated with both loss and gain of function of utx-1, we investigated the alternative lifespan pathways and tissue specificity of longevity outcomes. We demonstrated that lifespan extension caused by loss of utx-1 function is daf-16 dependent, while overexpression effects are partially independent of daf-16. In addition, lifespan extension was observed when utx-1 was knocked down or overexpressed in neurons and intestine, whereas in the epidermis, only knockdown of utx-1 conferred improved longevity.
Conclusions: We show that the regulation of longevity by chromatin modifiers can be the result of the interaction between distinct factors, such as the level and tissue of expression. Overall, we suggest that the heterochromatin loss model of ageing may be too simplistic an explanation of organismal ageing when molecular and tissue-specific effects are taken into account.
Keywords: Ageing; C. elegans; Chromatin; H3K27; Healthspan; Histone demethylase; Histone methyltransferase; Lifespan.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

