Natural killer cell therapy for hematologic malignancies: successes, challenges, and the future
- PMID: 33766099
- PMCID: PMC7992329
- DOI: 10.1186/s13287-021-02277-x
Natural killer cell therapy for hematologic malignancies: successes, challenges, and the future
Abstract
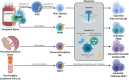
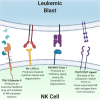
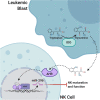
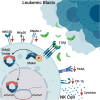
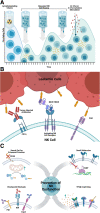
The adoptive transfer of natural killer (NK) cells is an emerging therapy in the field of immuno-oncology. In the last 3 decades, NK cells have been utilized to harness the anti-tumor immune response in a wide range of malignancies, most notably with early evidence of efficacy in hematologic malignancies. NK cells are dysfunctional in patients with hematologic malignancies, and their number and function are further impaired by chemotherapy, radiation, and immunosuppressants used in initial therapy and hematopoietic stem cell transplantation. Restoring this innate immune deficit may lead to improved therapeutic outcomes. NK cell adoptive transfer has proven to be a safe in these settings, even in the setting of HLA mismatch, and a deeper understanding of NK cell biology and optimized expansion techniques have improved scalability and therapeutic efficacy. Here, we review the use of NK cell therapy in hematologic malignancies and discuss strategies to further improve the efficacy of NK cells against these diseases.
Keywords: Cellular therapy; Hematologic malignancy; Natural killer cells.
Conflict of interest statement
D.A.L is on the scientific advisory board of the Caribou Biosciences and Courier Therapeutics. He is also on the scientific advisory board, provides consulting, owns stock, and has IP licensed to Kiadis Pharma.
B.P.T has IP licensed to Kiadis Pharma.
M.G.L and H.G.R have no conflicts of interest to disclose.
Figures

References
-
- Pizzolo G, Trentin L, Vinante F, Agostini C, Zambello R, Masciarelli M, Feruglio C, Dazzi F, Todeschini G, Chilosi M, Veneri D, Zanotti R, Benedetti F, Perona G, Semenzato G. Natural killer cell function and lymphoid subpopulations in acute non-lymphoblastic leukaemia in complete remission. Br J Cancer. 1988;58(3):368–372. doi: 10.1038/bjc.1988.221. - DOI - PMC - PubMed
-
- Khaznadar Z, Boissel N, Agaugué S, Henry G, Cheok M, Vignon M, Geromin D, Cayuela JM, Castaigne S, Pautas C, Raffoux E, Lachuer J, Sigaux F, Preudhomme C, Dombret H, Dulphy N, Toubert A. Defective NK cells in acute myeloid leukemia patients at diagnosis are associated with blast transcriptional signatures of immune evasion. J Immunol. 2015;195(6):2580–2590. doi: 10.4049/jimmunol.1500262. - DOI - PubMed
-
- Chretien AS, Fauriat C, Orlanducci F, Galseran C, Rey J, Bouvier Borg G, Gautherot E, Granjeaud S, Hamel-Broza JF, Demerle C, Ifrah N, Lacombe C, Cornillet-Lefebvre P, Delaunay J, Toubert A, Gregori E, Luche H, Malissen M, Arnoulet C, Nunes JA, Vey N, Olive D. Natural killer defective maturation is associated with adverse clinical outcome in patients with acute myeloid leukemia. Front Immunol. 2017;8:573. doi: 10.3389/fimmu.2017.00573. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

