Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies
- PMID: 33766109
- PMCID: PMC7993905
- DOI: 10.1186/s13054-021-03540-6
Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies
Abstract
Background: Although several international guidelines recommend early over late intubation of patients with severe coronavirus disease 2019 (COVID-19), this issue is still controversial. We aimed to investigate the effect (if any) of timing of intubation on clinical outcomes of critically ill patients with COVID-19 by carrying out a systematic review and meta-analysis.
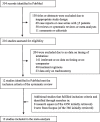
Methods: PubMed and Scopus were systematically searched, while references and preprint servers were explored, for relevant articles up to December 26, 2020, to identify studies which reported on mortality and/or morbidity of patients with COVID-19 undergoing early versus late intubation. "Early" was defined as intubation within 24 h from intensive care unit (ICU) admission, while "late" as intubation at any time after 24 h of ICU admission. All-cause mortality and duration of mechanical ventilation (MV) were the primary outcomes of the meta-analysis. Pooled risk ratio (RR), pooled mean difference (MD) and 95% confidence intervals (CI) were calculated using a random effects model. The meta-analysis was registered with PROSPERO (CRD42020222147).
Results: A total of 12 studies, involving 8944 critically ill patients with COVID-19, were included. There was no statistically detectable difference on all-cause mortality between patients undergoing early versus late intubation (3981 deaths; 45.4% versus 39.1%; RR 1.07, 95% CI 0.99-1.15, p = 0.08). This was also the case for duration of MV (1892 patients; MD - 0.58 days, 95% CI - 3.06 to 1.89 days, p = 0.65). In a sensitivity analysis using an alternate definition of early/late intubation, intubation without versus with a prior trial of high-flow nasal cannula or noninvasive mechanical ventilation was still not associated with a statistically detectable difference on all-cause mortality (1128 deaths; 48.9% versus 42.5%; RR 1.11, 95% CI 0.99-1.25, p = 0.08).
Conclusions: The synthesized evidence suggests that timing of intubation may have no effect on mortality and morbidity of critically ill patients with COVID-19. These results might justify a wait-and-see approach, which may lead to fewer intubations. Relevant guidelines may therefore need to be updated.
Keywords: Acute respiratory distress syndrome; Acute respiratory failure; Coronavirus; Delayed; Intensive care unit; Pneumonia.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


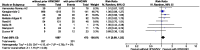
Comment in
-
Timing of Intubation in Covid-19 ARDS: What "time" really matters?Crit Care. 2021 May 21;25(1):173. doi: 10.1186/s13054-021-03598-2. Crit Care. 2021. PMID: 34020689 Free PMC article. No abstract available.
-
Timing of intubation in COVID-19: Not just location, location, location?Crit Care. 2021 Jun 4;25(1):193. doi: 10.1186/s13054-021-03617-2. Crit Care. 2021. PMID: 34088333 Free PMC article. No abstract available.
References
-
- Cook TM, El-Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesth Blackwell Publ. 2020;75:785–799. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

