Research diagnostic criteria for Alzheimer's disease: findings from the LipiDiDiet randomized controlled trial
- PMID: 33766132
- PMCID: PMC7995792
- DOI: 10.1186/s13195-021-00799-3
Research diagnostic criteria for Alzheimer's disease: findings from the LipiDiDiet randomized controlled trial
Abstract
Background: To explore the utility of the International Working Group (IWG)-1 criteria in recruitment for Alzheimer's disease (AD) clinical trials, we applied the more recently proposed research diagnostic criteria to individuals enrolled in a randomized controlled prevention trial (RCT) and assessed their disease progression.
Methods: The multinational LipiDiDiet RCT targeted 311 individuals with IWG-1 defined prodromal AD. Based on centrally analyzed baseline biomarkers, participants were classified according to the IWG-2 and National Institute on Aging-Alzheimer's Association (NIA-AA) 2011 and 2018 criteria. Linear mixed models were used to investigate the 2-year change in cognitive and functional performance (Neuropsychological Test Battery NTB Z scores, Clinical Dementia Rating-Sum of Boxes CDR-SB) (criteria × time interactions; baseline score, randomization group, sex, Mini-Mental State Examination (MMSE), and age also included in the models). Cox models adjusted for randomization group, MMSE, sex, age, and study site were used to investigate the risk of progression to dementia over 2 years.
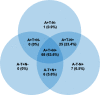
Results: In total, 88%, 86%, and 69% of participants had abnormal cerebrospinal fluid (CSF) β-amyloid, total tau, and phosphorylated tau, respectively; 64% had an A+T+N+ profile (CSF available for N = 107). Cognitive-functional decline appeared to be more pronounced in the IWG-2 prodromal AD, NIA-AA 2011 high and intermediate AD likelihood, and NIA-AA 2018 AD groups, but few significant differences were observed between the groups within each set of criteria. Hazard ratio (95% CI) for dementia was 4.6 (1.6-13.7) for IWG-2 prodromal AD (reference group no prodromal AD), 7.4 (1.0-54.7) for NIA-AA 2011 high AD likelihood (reference group suspected non-AD pathology SNAP), and 9.4 (1.2-72.7) for NIA-AA 2018 AD (reference group non-Alzheimer's pathologic change). Compared with the NIA-AA 2011 high AD likelihood group (abnormal β-amyloid and neuronal injury markers), disease progression was similar in the intermediate AD likelihood group (medial temporal lobe atrophy; no CSF available).
Conclusions: Despite being less restrictive than the other criteria, the IWG-1 criteria reliably identified individuals with AD pathology. More pragmatic and easily applicable selection criteria might be preferred due to feasibility in certain situations, e.g., in multidomain prevention trials that do not specifically target β-amyloid/tau pathologies.
Trial registration: Netherlands Trial Register, NL1620 . Registered on 9 March 2009.
Keywords: Alzheimer’s disease; Biomarkers; Cerebrospinal fluid; Disease progression; Early diagnosis; Prevention; Prodromal Alzheimer’s disease; Randomized controlled trial; Research criteria.
Conflict of interest statement
The LipiDiDiet consortium received funding by Danone Nutricia Research for the intervention period from 25 to 96 months, and the consortium distributed the funding to their members to conduct the trial and analysis. HS reports personal fees from ACI and MERCK (advisor), outside the submitted work. KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (disclosures not relevant for the submitted work). AR, AS, PJV, TH, and MK report no competing interests.
Figures

References
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. - DOI - PMC - PubMed
-
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

