Distinct tooth regeneration systems deploy a conserved battery of genes
- PMID: 33766133
- PMCID: PMC7995769
- DOI: 10.1186/s13227-021-00172-3
Distinct tooth regeneration systems deploy a conserved battery of genes
Abstract
Background: Vertebrate teeth exhibit a wide range of regenerative systems. Many species, including most mammals, reptiles, and amphibians, form replacement teeth at a histologically distinct location called the successional dental lamina, while other species do not employ such a system. Notably, a 'lamina-less' tooth replacement condition is found in a paraphyletic array of ray-finned fishes, such as stickleback, trout, cod, medaka, and bichir. Furthermore, the position, renewal potential, and latency times appear to vary drastically across different vertebrate tooth regeneration systems. The progenitor cells underlying tooth regeneration thus present highly divergent arrangements and potentials. Given the spectrum of regeneration systems present in vertebrates, it is unclear if morphologically divergent tooth regeneration systems deploy an overlapping battery of genes in their naïve dental tissues.
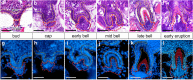
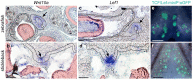
Results: In the present work, we aimed to determine whether or not tooth progenitor epithelia could be composed of a conserved cell type between vertebrate dentitions with divergent regeneration systems. To address this question, we compared the pharyngeal tooth regeneration processes in two ray-finned fishes: zebrafish (Danio rerio) and threespine stickleback (Gasterosteus aculeatus). These two teleost species diverged approximately 250 million years ago and demonstrate some stark differences in dental morphology and regeneration. Here, we find that the naïve successional dental lamina in zebrafish expresses a battery of nine genes (bmpr1aa, bmp6, cd34, gli1, igfbp5a, lgr4, lgr6, nfatc1, and pitx2), while active Wnt signaling and Lef1 expression occur during early morphogenesis stages of tooth development. We also find that, despite the absence of a histologically distinct successional dental lamina in stickleback tooth fields, the same battery of nine genes (Bmpr1a, Bmp6, CD34, Gli1, Igfbp5a, Lgr4, Lgr6, Nfatc1, and Pitx2) are expressed in the basalmost endodermal cell layer, which is the region most closely associated with replacement tooth germs. Like zebrafish, stickleback replacement tooth germs additionally express Lef1 and exhibit active Wnt signaling. Thus, two fish systems that either have an organized successional dental lamina (zebrafish) or lack a morphologically distinct successional dental lamina (sticklebacks) deploy similar genetic programs during tooth regeneration.
Conclusions: We propose that the expression domains described here delineate a highly conserved "successional dental epithelium" (SDE). Furthermore, a set of orthologous genes is known to mark hair follicle epithelial stem cells in mice, suggesting that regenerative systems in other epithelial appendages may utilize a related epithelial progenitor cell type, despite the highly derived nature of the resulting functional organs.
Keywords: Epithelial appendage; Odontode; Successional dental lamina; Tooth regeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Modulation of tooth regeneration through opposing responses to Wnt and BMP signals in teleosts.Development. 2023 Dec 1;150(23):dev202168. doi: 10.1242/dev.202168. Epub 2023 Dec 7. Development. 2023. PMID: 38059590 Free PMC article.
-
Formation of a successional dental lamina in the zebrafish (Danio rerio): support for a local control of replacement tooth initiation.Int J Dev Biol. 2006;50(7):637-43. doi: 10.1387/ijdb.052098ah. Int J Dev Biol. 2006. PMID: 16892177
-
Sox2+ progenitors in sharks link taste development with the evolution of regenerative teeth from denticles.Proc Natl Acad Sci U S A. 2016 Dec 20;113(51):14769-14774. doi: 10.1073/pnas.1612354113. Epub 2016 Dec 7. Proc Natl Acad Sci U S A. 2016. PMID: 27930309 Free PMC article.
-
Developmental pathways of periodontal tissue regeneration: Developmental diversities of tooth morphogenesis do also map capacity of periodontal tissue regeneration?J Periodontal Res. 2019 Feb;54(1):10-26. doi: 10.1111/jre.12596. Epub 2018 Sep 12. J Periodontal Res. 2019. PMID: 30207395 Review.
-
Reptilian tooth development.Genesis. 2011 Apr;49(4):247-60. doi: 10.1002/dvg.20721. Epub 2011 Apr 1. Genesis. 2011. PMID: 21309070 Review.
Cited by
-
Comparative transcriptome profiles of human dental pulp stem cells from maxillary and mandibular teeth.Sci Rep. 2022 May 25;12(1):8860. doi: 10.1038/s41598-022-12867-1. Sci Rep. 2022. PMID: 35614192 Free PMC article.
-
A Y-linked duplication of anti-Mullerian hormone is the sex determination gene in threespine stickleback.bioRxiv [Preprint]. 2025 Apr 29:2025.04.28.650899. doi: 10.1101/2025.04.28.650899. bioRxiv. 2025. PMID: 40463070 Free PMC article. Preprint.
-
Ectodysplasin overexpression reveals spatiotemporally dynamic tooth formation competency in stickleback and zebrafish.bioRxiv [Preprint]. 2025 May 7:2025.05.01.651241. doi: 10.1101/2025.05.01.651241. bioRxiv. 2025. PMID: 40654999 Free PMC article. Preprint.
-
Evolution of Spatial and Temporal cis-Regulatory Divergence in Sticklebacks.Mol Biol Evol. 2023 Mar 4;40(3):msad034. doi: 10.1093/molbev/msad034. Mol Biol Evol. 2023. PMID: 36805962 Free PMC article.
-
Site pleiotropy of a stickleback Bmp6 enhancer.Dev Biol. 2022 Dec;492:111-118. doi: 10.1016/j.ydbio.2022.09.012. Epub 2022 Oct 2. Dev Biol. 2022. PMID: 36198347 Free PMC article.
References
-
- Sasagawa I, Ishiyama M, Yokosuka H, Mikami M, Uchida T. Tooth enamel and enameloid in actinopterygian fish. Front Mater Sci China. 2009;3:174. doi: 10.1007/s11706-009-0030-3. - DOI
-
- Smith MM, Hall BK. A developmental model for evolution of the vertebrate exoskeleton and teeth. In: Hecht MK, MacIntyre RJ, Clegg MT, editors. Evolutionary biology. Boston: Springer; 1993. pp. 387–448.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

