Serological surveillance of SARS-CoV-2: Six-month trends and antibody response in a cohort of public health workers
- PMID: 33766553
- PMCID: PMC7982645
- DOI: 10.1016/j.jinf.2021.03.015
Serological surveillance of SARS-CoV-2: Six-month trends and antibody response in a cohort of public health workers
Abstract
Background: Antibody waning after SARS-CoV-2 infection may result in reduction in long-term immunity following natural infection and vaccination, and is therefore a major public health issue. We undertook prospective serosurveillance in a large cohort of healthy adults from the start of the epidemic in England.
Methods: Clinical and non-clinical healthcare workers were recruited across three English regions and tested monthly from March to November 2020 for SARS-CoV-2 spike (S) protein and nucleoprotein (N) antibodies using five different immunoassays. In positive individuals, antibody responses and long-term trends were modelled using mixed effects regression.
Findings: In total, 2246 individuals attended 12,247 visits and 264 were seropositive in ≥ 2 assays. Most seroconversions occurred between March and April 2020. The assays showed > 85% agreement for ever-positivity, although this changed markedly over time. Antibodies were detected earlier with Abbott (N) but declined rapidly thereafter. With the EuroImmun (S) and receptor-binding domain (RBD) assays, responses increased for 4 weeks then fell until week 12-16 before stabilising. For Roche (N), responses increased until 8 weeks, stabilised, then declined, but most remained above the positive threshold. For Roche (S), responses continued to climb over the full 24 weeks, with no sero-reversions. Predicted proportions sero-reverting after 52 weeks were 100% for Abbott, 59% (95% credible interval 50-68%) Euroimmun, 41% (30-52%) RBD, 10% (8-14%) Roche (N) < 2% Roche (S).
Interpretation: Trends in SARS-CoV-2 antibodies following infection are highly dependent on the assay used. Ongoing serosurveillance using multiple assays is critical for monitoring the course and long-term progression of SARS-CoV-2 antibodies.
Copyright © 2021. Published by Elsevier Ltd.
Figures
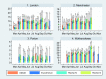
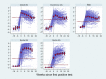
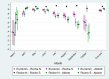
Comment in
-
Reactogenicity, safety and antibody response, after one and two doses of mRNA-1273 in seronegative and seropositive healthcare workers.J Infect. 2021 Aug;83(2):237-279. doi: 10.1016/j.jinf.2021.03.025. Epub 2021 Apr 1. J Infect. 2021. PMID: 33811939 Free PMC article. No abstract available.
-
One-year durability of anti-spike IgG to SARS-CoV-2: Preliminary data from the anticrown prospective observational study one year durability of COVID-19 anti-spike IgG.J Infect. 2021 Aug;83(2):237-279. doi: 10.1016/j.jinf.2021.05.023. Epub 2021 May 27. J Infect. 2021. PMID: 34052240 Free PMC article.
-
Decreasing humoral response among healthcare workers up to 4 months after two doses of BNT162b2 vaccine.J Infect. 2022 Feb;84(2):248-288. doi: 10.1016/j.jinf.2021.09.017. Epub 2021 Sep 29. J Infect. 2022. PMID: 34600020 Free PMC article. No abstract available.
References
-
- He J, Guo Y, Mao R, Zhang J. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J Med Virol. 2020 http://www.ncbi.nlm.nih.gov/pubmed/32691881 Jul 21Available from. - PMC - PubMed
-
- Wu X, Liu L, Jiao J, Yang L, Zhu B, Li X. Characterisation of clinical, laboratory and imaging factors related to mild vs. severe covid-19 infection: a systematic review and meta-analysis. Ann Med. 2020:1–11. http://www.ncbi.nlm.nih.gov/pubmed/32755287 Aug 11Available from. - PMC - PubMed
-
- Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, et al. Clinical features of COVID-19 and factors associated with severe clinical course: a systematic review and meta-analysis. SSRN. 2020 http://www.ncbi.nlm.nih.gov/pubmed/32714109 Apr 21Available from. - PMC - PubMed
-
- Iversen K, Bundgaard H, Hasselbalch RB, Kristensen JH, Nielsen PB, Pries-Heje M, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020 http://www.ncbi.nlm.nih.gov/pubmed/32758438 Aug 30(0). Available from. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

