A Randomized Trial of Tenapanor and Phosphate Binders as a Dual-Mechanism Treatment for Hyperphosphatemia in Patients on Maintenance Dialysis (AMPLIFY)
- PMID: 33766811
- PMCID: PMC8259655
- DOI: 10.1681/ASN.2020101398
A Randomized Trial of Tenapanor and Phosphate Binders as a Dual-Mechanism Treatment for Hyperphosphatemia in Patients on Maintenance Dialysis (AMPLIFY)
Abstract
Background: Hyperphosphatemia is associated with cardiovascular morbidity and mortality in patients receiving maintenance dialysis. It is unknown whether combining two therapies with different mechanisms of action-tenapanor, an inhibitor of paracellular phosphate absorption, and phosphate binders-is safe and effective for the management of hyperphosphatemia in patients receiving maintenance dialysis.
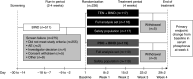
Methods: This double-blind phase 3 trial enrolled 236 patients undergoing maintenance dialysis with hyperphosphatemia (defined in this trial as serum phosphorus 5.5-10 mg/dl inclusive) despite receiving phosphate binder therapy (sevelamer, nonsevelamer, sevelamer plus nonsevelamer, or multiple nonsevelamer binders). These participants were randomly assigned to receive oral tenapanor 30 mg twice daily or placebo for 4 weeks. The primary efficacy end point was the change in serum phosphorus concentration from baseline to week 4.
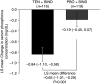
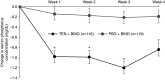
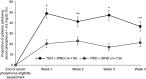
Results: Of the 236 randomized patients, 235 (99.6%) were included in the full analysis set; this included 116 in the tenapanor plus binder group and 119 in the placebo plus binder group. A total of 228 patients (96.6%) completed the 4-week treatment period. In the full analysis set (mean age 54.5 years, 40.9% women), patients treated with tenapanor plus binder achieved a larger mean change in serum phosphorus concentration from baseline to week 4 compared with placebo plus binder (-0.84 versus -0.19 mg/dl, P<0.001). Diarrhea was the most commonly reported adverse event, resulting in study drug discontinuation in four of 119 (3.4%) and two of 116 (1.7%) patients receiving tenapanor plus binder or placebo plus binder, respectively.
Conclusions: A dual-mechanism treatment using both tenapanor and phosphate binders improved control of hyperphosphatemia in patients undergoing maintenance dialysis compared with phosphate binders alone.
Clinical trial registry name and registration number: AMPLIFY, NCT03824587.
Keywords: FGF23; NHE3 dialysis; hyperphosphatemia; phosphate binders; phosphate uptake; phosphorus; tenapanor.
Copyright © 2021 by the American Society of Nephrology.
Figures

Similar articles
-
Therapeutic Effects of Add-On Tenapanor for Hemodialysis Patients with Refractory Hyperphosphatemia.Am J Nephrol. 2021;52(6):496-506. doi: 10.1159/000516156. Epub 2021 Jun 7. Am J Nephrol. 2021. PMID: 34098559 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Tenapanor in Patients with Hyperphosphatemia Receiving Maintenance Hemodialysis: A Randomized Phase 3 Trial.J Am Soc Nephrol. 2019 Apr;30(4):641-652. doi: 10.1681/ASN.2018080832. Epub 2019 Mar 7. J Am Soc Nephrol. 2019. PMID: 30846557 Free PMC article. Clinical Trial.
-
Effectivity and safety profile of tenapanor, a sodium-hydrogen exchanger isoform 3 inhibitor, as an innovative treatment for hyperphosphatemia in chronic kidney disease: A systematic review of clinical studies.Nefrologia (Engl Ed). 2024 Nov-Dec;44(6):796-806. doi: 10.1016/j.nefroe.2024.11.015. Nefrologia (Engl Ed). 2024. PMID: 39701606
-
Safety and Efficacy of Tenapanor for Long-term Serum Phosphate Control in Maintenance Dialysis: A 52-Week Randomized Phase 3 Trial (PHREEDOM).Kidney360. 2021 Aug 27;2(10):1600-1610. doi: 10.34067/KID.0002002021. eCollection 2021 Oct 28. Kidney360. 2021. PMID: 35372979 Free PMC article. Clinical Trial.
-
Tenapanor: A Phosphate Absorption Inhibitor for the Management of Hyperphosphatemia in Patients With Kidney Failure.J Ren Nutr. 2025 Jan;35(1):25-34. doi: 10.1053/j.jrn.2024.07.003. Epub 2024 Jul 9. J Ren Nutr. 2025. PMID: 38992521 Review.
Cited by
-
Efficacy of tenapanor in managing hyperphosphatemia and constipation in hemodialysis patients: A randomized controlled trial.PLoS One. 2025 Jun 17;20(6):e0319319. doi: 10.1371/journal.pone.0319319. eCollection 2025. PLoS One. 2025. PMID: 40526762 Free PMC article. Clinical Trial.
-
Past, Present, and Future of Phosphate Management.Kidney Int Rep. 2022 Feb 1;7(4):688-698. doi: 10.1016/j.ekir.2022.01.1055. eCollection 2022 Apr. Kidney Int Rep. 2022. PMID: 35497793 Free PMC article. Review.
-
The open system of FGF-23 at the crossroad between additional P-lowering therapy, anemia and inflammation: how to deal with the intact and the C-terminal assays?Clin Kidney J. 2023 Jun 27;16(10):1543-1549. doi: 10.1093/ckj/sfad144. eCollection 2023 Oct. Clin Kidney J. 2023. PMID: 37779858 Free PMC article.
-
Emerging cross-talks between chronic kidney disease-mineral and bone disorder (CKD-MBD) and malnutrition-inflammation complex syndrome (MICS) in patients receiving dialysis.Clin Exp Nephrol. 2022 Jul;26(7):613-629. doi: 10.1007/s10157-022-02216-x. Epub 2022 Mar 30. Clin Exp Nephrol. 2022. PMID: 35353283 Free PMC article. Review.
-
Hyperphosphatemia in Chronic Kidney Disease: The Search for New Treatment Paradigms and the Role of Tenapanor.Int J Nephrol Renovasc Dis. 2024 May 28;17:151-161. doi: 10.2147/IJNRD.S385826. eCollection 2024. Int J Nephrol Renovasc Dis. 2024. PMID: 38831770 Free PMC article. Review.
References
-
- Rastogi A, Bhatt N, Rossetti S, Beto J: Management of hyperphosphatemia in end-stage renal disease: A new paradigm. J Ren Nutr 31: 21–34, 2021. - PubMed
-
- Mizobuchi M, Towler D, Slatopolsky E: Vascular calcification: The killer of patients with chronic kidney disease. J Am Soc Nephrol 20: 1453–1464, 2009. - PubMed
-
- Sigrist MK, Taal MW, Bungay P, McIntyre CW: Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol 2: 1241–1248, 2007. - PubMed
-
- Cano-Megías M, Guisado-Vasco P, Bouarich H, de Arriba-de la Fuente G, de Sequera-Ortiz P, Álvarez-Sanz C, et al. .: Coronary calcification as a predictor of cardiovascular mortality in advanced chronic kidney disease: A prospective long-term follow-up study. BMC Nephrol 20: 188, 2019. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

