Interim Analysis of the Phase II Study: Noninferiority Study of Doxorubicin with Upfront Dexrazoxane plus Olaratumab for Advanced or Metastatic Soft-Tissue Sarcoma
- PMID: 33766818
- PMCID: PMC8282681
- DOI: 10.1158/1078-0432.CCR-20-4621
Interim Analysis of the Phase II Study: Noninferiority Study of Doxorubicin with Upfront Dexrazoxane plus Olaratumab for Advanced or Metastatic Soft-Tissue Sarcoma
Abstract
Purpose: To report the interim analysis of the phase II single-arm noninferiority trial, testing the upfront use of dexrazoxane with doxorubicin on progression-free survival (PFS) and cardiac function in soft-tissue sarcoma (STS).
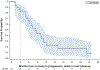
Patients and methods: Patients with metastatic or unresectable STS who were candidates for first-line treatment with doxorubicin were deemed eligible. An interim analysis was initiated after 33 of 65 patients were enrolled. Using the historical control of 4.6 months PFS for doxorubicin in the front-line setting, we tested whether the addition of dexrazoxane affected the efficacy of doxorubicin in STS. The study was powered so that a decrease of PFS to 3.7 months would be considered noninferior. Secondary aims included cardiac-related mortality, incidence of heart failure/cardiomyopathy, and expansion of cardiac monitoring parameters including three-dimensional echocardiography. Patients were allowed to continue on doxorubicin beyond 600 mg/m2 if they were deriving benefit and were not demonstrating evidence of symptomatic cardiac dysfunction.
Results: At interim analysis, upfront use of dexrazoxane with doxorubicin demonstrated a PFS of 8.4 months (95% confidence interval: 5.1-11.2 months). Only 3 patients were removed from study for cardiotoxicity, all on > 600 mg/m2 doxorubicin. No patients required cardiac hospitalization or had new, persistent cardiac dysfunction with left ventricular ejection fraction remaining below 50%. The median administered doxorubicin dose was 450 mg/m2 (interquartile range, 300-750 mg/m2).
Conclusions: At interim analysis, dexrazoxane did not reduce PFS in patients with STS treated with doxorubicin. Involvement of cardio-oncologists is beneficial for the monitoring and safe use of high-dose anthracyclines in STS.See related commentary by Benjamin and Minotti, p. 3809.
©2021 American Association for Cancer Research.
Conflict of interest statement
Conflicts of Interest
Brian A. Van Tine: grants from Merck; grants and personal fees from Pfizer; grants from TRACON Pharmaceuticals; grants, personal fees, and other from GlaxoSmithKline; personal fees from Polaris Inc.; personal fees from Lilly; personal fees from Caris Life Sciences; personal fees from Novartis; personal fees from CytRX; personal fees from Plexxikon; personal fees from Epizyme; personal fees from Daiichi Sankyo; personal fees from Adaptimmune; personal fees from Immune Design; personal fees from Bayer; personal fees from Cytokinetics; personal fees from Deciphera; and has a patent issued for the use of ME1 as a biomarker and ACXT3102.
Angela C. Hirbe: consult to Astrazeneca and Springworks
Peter Oppelt: speaking fees/honoraria: Merck, Bristol-Myers, Eisai
Ashley E. Frith: none
Richa Rathore: none
Joshua D. Mitchell: consultant to Pfizer.
Fei Wan: none
Shellie Berry: none
Michele Landeau: none
George A. Heberton: none
John Gorcsan III: research grants for GE, Canon, V-wave and EBR systems
Peter R. Huntjens: none
Yuko Soyama: none
Justin M. Vader: none
Jose A. Alvarez Cardona: none
Kathleen W. Zhang: consultant fees from Eidos Therapeutics
Daniel J. Lenihan: consultant to Roche, Prothena, Lilly Clementia and Reseach Funding from Myocardial Solutions
Ronald J. Krone: none
Figures

Comment in
-
Doxorubicin-Dexrazoxane from Day 1 for Soft-tissue Sarcomas: The Road to Cardioprotection.Clin Cancer Res. 2021 Jul 15;27(14):3809-3811. doi: 10.1158/1078-0432.CCR-21-1376. Epub 2021 May 14. Clin Cancer Res. 2021. PMID: 33990361
Similar articles
-
Dexrazoxane for preventing or reducing cardiotoxicity in adults and children with cancer receiving anthracyclines.Cochrane Database Syst Rev. 2022 Sep 27;9(9):CD014638. doi: 10.1002/14651858.CD014638.pub2. Cochrane Database Syst Rev. 2022. PMID: 36162822 Free PMC article.
-
Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2. Cochrane Database Syst Rev. 2017. PMID: 28901021 Free PMC article.
-
Autologous hematopoietic stem cell transplantation following high dose chemotherapy for non-rhabdomyosarcoma soft tissue sarcomas.Cochrane Database Syst Rev. 2013 Aug 7;2013(8):CD008216. doi: 10.1002/14651858.CD008216.pub4. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Apr 13;4:CD008216. doi: 10.1002/14651858.CD008216.pub5. PMID: 23925699 Free PMC article. Updated.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
Cited by
-
Dexrazoxane makes doxorubicin-induced heart failure a rare event in sarcoma patients receiving high cumulative doses.Cardiooncology. 2025 Mar 19;11(1):29. doi: 10.1186/s40959-025-00323-8. Cardiooncology. 2025. PMID: 40108682 Free PMC article. Review.
-
Fatal heart disease in patients with bone and soft tissue sarcoma.Front Cardiovasc Med. 2022 Oct 13;9:951940. doi: 10.3389/fcvm.2022.951940. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36312272 Free PMC article.
-
How to utilize current guidelines to manage patients with cancer at high risk for heart failure.Cardiooncology. 2024 Sep 28;10(1):63. doi: 10.1186/s40959-024-00259-5. Cardiooncology. 2024. PMID: 39342407 Free PMC article. Review.
-
Cardiac events among patients with sarcoma treated with doxorubicin by method of infusion: A real-world database study.Cancer Rep (Hoboken). 2023 Jan;6(1):e1681. doi: 10.1002/cnr2.1681. Epub 2022 Jul 18. Cancer Rep (Hoboken). 2023. PMID: 35852051 Free PMC article.
-
Prevention of Heart Failure Induced by Doxorubicin with Early Administration of Dexrazoxane (PHOENIX Study): dose response and time course of dexrazoxane-induced degradation of topoisomerase 2b.Cardiooncology. 2025 May 2;11(1):42. doi: 10.1186/s40959-025-00339-0. Cardiooncology. 2025. PMID: 40317097 Free PMC article.
References
-
- Tap WD, Wagner AJ, Schöffski P, Martin-Broto J, Krarup-Hansen A, Ganjoo KN, et al. Effect of Doxorubicin Plus Olaratumab vs Doxorubicin Plus Placebo on Survival in Patients With Advanced Soft Tissue Sarcomas: The ANNOUNCE Randomized Clinical Trial. Jama 2020;323(13):1266–76 doi 10.1001/jama.2020.1707. - DOI - PMC - PubMed
-
- Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J 2016;37(21):1671–80 doi 10.1093/eurheartj/ehw022. - DOI - PMC - PubMed
-
- Bosch X, Rovira M, Sitges M, Domenech A, Ortiz-Perez JT, de Caralt TM, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). Journal of the American College of Cardiology 2013;61(23):2355–62 doi 10.1016/j.jacc.2013.02.072. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

