Protocol for a randomised controlled trial comparing laparoscopic cholecystectomy with observation/conservative management for preventing recurrent symptoms and complications in adults with uncomplicated symptomatic gallstones (C-Gall trial)
- PMID: 33766835
- PMCID: PMC7996370
- DOI: 10.1136/bmjopen-2020-039781
Protocol for a randomised controlled trial comparing laparoscopic cholecystectomy with observation/conservative management for preventing recurrent symptoms and complications in adults with uncomplicated symptomatic gallstones (C-Gall trial)
Abstract
Background: Gallstone disease (cholelithiasis) is common. In most people it is asymptomatic and does not require treatment, but in about 20% it can become symptomatic, causing pain and other complications requiring medical attention and/or surgery. A proportion of symptomatic people with uncomplicated gallstone disease do not experience further episodes of pain and, therefore, could be treated conservatively. Moreover, surgery carries risks of perioperative and postoperative complications.
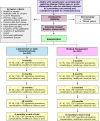
Methods and analysis: C-Gall is a pragmatic, multicentre, randomised controlled trial and economic evaluation to assess whether cholecystectomy is cost-effective compared with observation/ conservative management (here after referred to as medical management) at 18 months post-randomisation (with internal pilot).
Primary outcome measure: Patient-reported quality of life (QoL) (36-Item Short Form Survey (SF-36) bodily pain domain) up to 18 months after randomisation.The primary economic outcome is incremental cost per quality-adjusted life year gained at 18 months.
Secondary outcome measures: Secondary outcome measures include condition-specific QoL, SF-36 domains, complications, further treatment, persistent symptoms, healthcare resource use, and costs assessed at 18 and 24 months after randomisation. The bodily pain domain of the SF-36 will also be assessed at 24 months after randomisation.A sample size of 430 participants was calculated. Computer-generated 1:1 randomisation was used.The C-Gall Study is currently in follow-up in 20 UK research centres. The first patient was randomised on 1 August 2016, with follow-up to be completed by 30 November 2021.
Statistical analysis: Statistical analysis of the primary outcome will be intention-to-treat and a per-protocol analysis. The primary outcome, area under the curve (AUC) for the SF-36 bodily pain up to 18 months, will be generated using the Trapezium rule and analysed using linear regression with adjustment for the minimisation variables (recruitment site, sex and age). For the secondary outcome, SF-36 bodily pain, AUC up to 24 months will be analysed in a similar way. Other secondary outcomes will be analysed using generalised linear models with adjustment for minimisation and baseline variables, as appropriate. Statistical significance will be at the two-sided 5% level with corresponding CIs.
Ethics and dissemination: The North of Scotland Research Ethics Committee approved this study (16/NS/0053). The dissemination plans include Health Technology Assessment monograph, international scientific meetings and publications in high-impact, open-access journals.
Trial registration number: ISRCTN55215960; pre-results.
Keywords: adult gastroenterology; gastroenterology; protocols & guidelines; surgery.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous