mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection
- PMID: 33766944
- PMCID: PMC8139425
- DOI: 10.1126/science.abg9175
mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection
Abstract
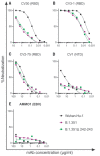
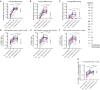
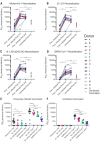
Emerging SARS-CoV-2 variants have raised concerns about resistance to neutralizing antibodies elicited by previous infection or vaccination. We examined whether sera from recovered and naïve donors collected prior to, and following immunizations with existing mRNA vaccines, could neutralize the Wuhan-Hu-1 and B.1.351 variants. Pre-vaccination sera from recovered donors neutralized Wuhan-Hu-1 and sporadically neutralized B.1.351, but a single immunization boosted neutralizing titers against all variants and SARS-CoV-1 by up to 1000-fold. Neutralization was due to antibodies targeting the receptor binding domain and was not boosted by a second immunization. Immunization of naïve donors also elicited cross-neutralizing responses, but at lower titers. Our study highlights the importance of vaccinating both uninfected and previously infected persons to elicit cross-variant neutralizing antibodies.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures

Update of
-
A single mRNA immunization boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection.medRxiv [Preprint]. 2021 Mar 10:2021.02.05.21251182. doi: 10.1101/2021.02.05.21251182. medRxiv. 2021. Update in: Science. 2021 Mar 25:eabg9175. doi: 10.1126/science.abg9175. PMID: 33758873 Free PMC article. Updated. Preprint.
Comment in
-
Variant constraint by mRNA vaccines.Nat Rev Immunol. 2021 May;21(5):274-275. doi: 10.1038/s41577-021-00548-5. Nat Rev Immunol. 2021. PMID: 33837367 Free PMC article.
-
Catch-as-catch-can: mRNA vaccination boosts immune responses to SARS-CoV-2 variants.Signal Transduct Target Ther. 2021 Jul 7;6(1):259. doi: 10.1038/s41392-021-00681-6. Signal Transduct Target Ther. 2021. PMID: 34234098 Free PMC article. No abstract available.
References
-
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L., A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020). 10.1038/s41586-020-2012-7 - DOI - PMC - PubMed
-
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G. F., Tan W., China Novel Coronavirus Investigating and Research Team , A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733 (2020). 10.1056/NEJMoa2001017 - DOI - PMC - PubMed
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T. S., Herrler G., Wu N.-H., Nitsche A., Müller M. A., Drosten C., Pöhlmann S., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181, 271–280.e8 (2020). 10.1016/j.cell.2020.02.052 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

