Intravenous Immunoglobulin Therapy in Patients With Painful Idiopathic Small Fiber Neuropathy
- PMID: 33766992
- PMCID: PMC8205474
- DOI: 10.1212/WNL.0000000000011919
Intravenous Immunoglobulin Therapy in Patients With Painful Idiopathic Small Fiber Neuropathy
Abstract
Objective: This is the first double-blind randomized controlled trial evaluating the efficacy and safety of IV immunoglobulin (IVIG) vs placebo in patients with idiopathic small fiber neuropathy (I-SFN).
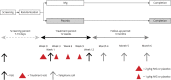
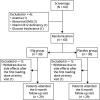
Methods: Between July 2016 and November 2018, 60 Dutch patients with skin biopsy-proven I-SFN randomly received a starting dose of IVIG (2 g/kg body weight) or matching placebo (0.9% saline). Subsequently, 3 additional infusions of IVIG (1 g/kg) or placebo were administered at 3-week intervals. The primary outcome was a 1-point change in Pain Intensity Numerical Rating Scale score at 12 weeks compared to baseline.
Results: Thirty patients received IVIG, and 30 received placebo. In both groups, 29 patients completed the trial. In 40% of patients receiving IVIG, the mean average pain was decreased by at least 1 point compared to 30% of the patients receiving placebo (p = 0.588, odds ratio 1.56, 95% confidence interval 0.53-4.53). No significant differences were found on any of the other prespecified outcomes, including general well-being, autonomic symptoms, and overall functioning and disability.
Conclusions: This randomized controlled trial showed that IVIG treatment had no significant effect on pain in patients with painful I-SFN.
Trial registration information: ClinicalTrials.gov Identifier: NCT02637700, EudraCT 2015-002624-31.
Classification of evidence: This study provides Class I evidence that for patients with painful I-SFN, IVIG did not significantly reduce pain compared to placebo.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
Comment in
-
IVIG and Small Fiber Neuropathy: The Ongoing Search for Evidence.Neurology. 2021 May 18;96(20):929-930. doi: 10.1212/WNL.0000000000011921. Epub 2021 Mar 25. Neurology. 2021. PMID: 33766998 No abstract available.
-
Reader Response: Intravenous Immunoglobulin Therapy in Patients With Painful Idiopathic Small Fiber Neuropathy.Neurology. 2021 Oct 19;97(16):791-792. doi: 10.1212/WNL.0000000000012711. Neurology. 2021. PMID: 34663740 No abstract available.
-
Author Response: Intravenous Immunoglobulin Therapy in Patients With Painful Idiopathic Small Fiber Neuropathy.Neurology. 2021 Oct 19;97(16):792. doi: 10.1212/WNL.0000000000012712. Neurology. 2021. PMID: 34663741 No abstract available.
-
Reader Response: Intravenous Immunoglobulin Therapy in Patients With Painful Idiopathic Small Fiber Neuropathy.Neurology. 2021 Oct 19;97(16):793. doi: 10.1212/WNL.0000000000012709. Neurology. 2021. PMID: 34663742 No abstract available.
-
Author Response: Intravenous Immunoglobulin Therapy in Patients With Painful Idiopathic Small Fiber Neuropathy.Neurology. 2021 Oct 19;97(16):793-794. doi: 10.1212/WNL.0000000000012710. Neurology. 2021. PMID: 34663743 No abstract available.
-
Reader Response: Intravenous Immunoglobulin Therapy in Patients With Painful Idiopathic Small Fiber Neuropathy.Neurology. 2021 Oct 19;97(16):794. doi: 10.1212/WNL.0000000000012713. Neurology. 2021. PMID: 34663744 No abstract available.
-
Author Response: Intravenous Immunoglobulin Therapy in Patients With Painful Idiopathic Small Fiber Neuropathy.Neurology. 2021 Oct 19;97(16):794-795. doi: 10.1212/WNL.0000000000012714. Neurology. 2021. PMID: 34663745 No abstract available.
-
Follow-up Reader Response: Intravenous Immunoglobulin Therapy in Patients With Painful Idiopathic Small Fiber Neuropathy.Neurology. 2022 Jan 18;98(3):128-129. doi: 10.1212/WNL.0000000000013076. Neurology. 2022. PMID: 35039457 No abstract available.
-
Follow-up Author Response: Intravenous Immunoglobulin Therapy in Patients With Painful Idiopathic Small Fiber Neuropathy.Neurology. 2022 Jan 18;98(3):129-130. doi: 10.1212/WNL.0000000000013083. Neurology. 2022. PMID: 35039458 No abstract available.
-
Reader Response: Intravenous Immunoglobulin Therapy in Patients With Painful Idiopathic Small-Fiber Neuropathy.Neurology. 2022 Oct 11;99(15):675-676. doi: 10.1212/WNL.0000000000201313. Neurology. 2022. PMID: 36216520 No abstract available.
-
Author Response: Intravenous Immunoglobulin Therapy in Patients With Painful Idiopathic Small Fiber Neuropathy.Neurology. 2022 Oct 11;99(15):676-677. doi: 10.1212/WNL.0000000000201314. Neurology. 2022. PMID: 36216524 No abstract available.
References
-
- Hoeijmakers JG, Faber CG, Lauria G, Merkies IS, Waxman SG. Small-fibre neuropathies-advances in diagnosis, pathophysiology and management. Nat Rev Neurol 2012;8:369–379. - PubMed
-
- Cazzato D, Lauria G. Small fibre neuropathy. Curr Opin Neurol 2017;30:490–499. - PubMed
-
- Hoitsma E, Marziniak M, Faber CG, et al. Small fibre neuropathy in sarcoidosis. Lancet 2002;359:2085–2086. - PubMed
-
- Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003;60:108–111. - PubMed
-
- Brannagan TH III, Hays AP, Chin SS, et al. Small-fiber neuropathy/neuronopathy associated with celiac disease: skin biopsy findings. Arch Neurol 2005;62:1574–1578. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
