The critical role of FXR is associated with the regulation of autophagy and apoptosis in the progression of AKI to CKD
- PMID: 33767132
- PMCID: PMC7994637
- DOI: 10.1038/s41419-021-03620-z
The critical role of FXR is associated with the regulation of autophagy and apoptosis in the progression of AKI to CKD
Abstract
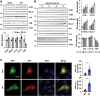
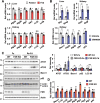
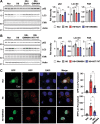
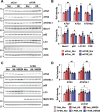
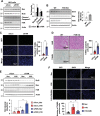
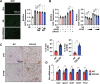
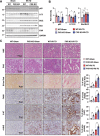
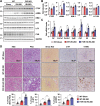
Autophagy is important for cells to break down and recycle cellular proteins, remove damaged organelles, and especially, for recovery from acute kidney injury (AKI). Despite research on the role and cellular mechanism of autophagy in AKI, the role of autophagy in the progression to chronic kidney disease (CKD) remains poorly understood. Here, using farnesoid X receptor (FXR) knockout (KO) mice, we determined whether FXR prevents the progression of AKI to CKD after renal ischemic-reperfusion (such as I/R) injury through the regulation of renal autophagy and apoptosis. FXR regulated genes that participate in renal autophagy under feeding and fasting conditions, such as hepatic autophagy, and the activation of FXR by agonists, such as GW4064 and INT-747, attenuated the increased autophagy and apoptosis of hypoxia-induced human renal proximal tubule epithelial (HK2) cells. The expression levels of autophagy-related and apoptosis-related proteins in FXR KO mice were increased compared with those in wild-type (WT) mice. We also showed that the increase in reactive oxidative species (ROS) in hypoxia-treated HK2 cells was attenuated by treatment with FXR agonist or by FXR overexpression, and that the level of ROS was elevated in FXR-deficient cells and mice. At 28 days after I/R injury, the autophagy levels were still elevated in FXR KO mice, and the expression levels of fibrosis-related proteins and ROS deposits were higher than those in WT mice. In conclusion, the regulation of renal autophagy and apoptosis by FXR may be a therapeutic target for the early stages of kidney damage, and the progression of AKI to CKD.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Knockout of farnesoid X receptor gene aggravates cisplatin-induced kidney injury.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022 Feb 28;47(2):174-182. doi: 10.11817/j.issn.1672-7347.2022.210423. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 35545407 Free PMC article. Chinese, English.
-
A naturally occurring FXR agonist, alisol B 23-acetate, protects against renal ischemia-reperfusion injury.Am J Physiol Renal Physiol. 2021 Nov 1;321(5):F617-F628. doi: 10.1152/ajprenal.00193.2021. Epub 2021 Sep 27. Am J Physiol Renal Physiol. 2021. PMID: 34569253
-
Farnesoid X receptor promotes renal ischaemia-reperfusion injury by inducing tubular epithelial cell apoptosis.Cell Prolif. 2021 Apr;54(4):e13005. doi: 10.1111/cpr.13005. Epub 2021 Feb 16. Cell Prolif. 2021. PMID: 33594777 Free PMC article.
-
Autophagy in acute kidney injury and repair.Nephron Clin Pract. 2014;127(1-4):56-60. doi: 10.1159/000363677. Epub 2014 Sep 24. Nephron Clin Pract. 2014. PMID: 25343822 Free PMC article. Review.
-
Autophagy and Acute Kidney Injury.Adv Exp Med Biol. 2020;1207:469-480. doi: 10.1007/978-981-15-4272-5_34. Adv Exp Med Biol. 2020. PMID: 32671769 Review.
Cited by
-
Bile acids and salt-sensitive hypertension: a role of the gut-liver axis.Am J Physiol Heart Circ Physiol. 2022 Apr 1;322(4):H636-H646. doi: 10.1152/ajpheart.00027.2022. Epub 2022 Mar 4. Am J Physiol Heart Circ Physiol. 2022. PMID: 35245132 Free PMC article. Review.
-
LncRNA136131 suppresses apoptosis of renal tubular epithelial cells in acute kidney injury by targeting the miR-378a-3p/Rab10 axis.Aging (Albany NY). 2022 Apr 28;14(8):3666-3686. doi: 10.18632/aging.204036. Epub 2022 Apr 28. Aging (Albany NY). 2022. PMID: 35482482 Free PMC article.
-
Investigating the Mechanisms of Jieduquyuziyin Prescription Improves Lupus Nephritis and Fibrosis via FXR in MRL/lpr Mice.Oxid Med Cell Longev. 2022 Jul 9;2022:4301033. doi: 10.1155/2022/4301033. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35855861 Free PMC article.
-
Protective effects of mefunidone on ischemia-reperfusion injury/Folic acid-induced acute kidney injury.Front Pharmacol. 2022 Nov 23;13:1043945. doi: 10.3389/fphar.2022.1043945. eCollection 2022. Front Pharmacol. 2022. PMID: 36506525 Free PMC article.
-
Autophagy in acute kidney injury and maladaptive kidney repair.Burns Trauma. 2023 Jan 22;11:tkac059. doi: 10.1093/burnst/tkac059. eCollection 2023. Burns Trauma. 2023. PMID: 36694860 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

