Electrocatalytic synthesis of heterocycles from biomass-derived furfuryl alcohols
- PMID: 33767166
- PMCID: PMC7994825
- DOI: 10.1038/s41467-021-22157-5
Electrocatalytic synthesis of heterocycles from biomass-derived furfuryl alcohols
Abstract
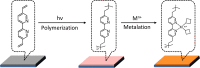
It is very attractive yet underexplored to synthesize heterocyclic moieties pertaining to biologically active molecules from biomass-based starting compounds. Herein, we report an electrocatalytic Achmatowicz reaction for the synthesis of hydropyranones from furfuryl alcohols, which can be readily produced from biomass-derived and industrially available furfural. Taking advantage of photo-induced polymerization of a bipyridyl ligand, we demonstrate the facile preparation of a heterogenized nickel electrocatalyst, which effectively drives the Achmatowicz reaction electrochemically. A suite of characterization techniques and density functional theory computations were performed to aid the understanding of the reaction mechanism. It is rationalized that the unsaturated coordination sphere of nickel sites in our electrocatalyst plays an important role at low applied potential, not only allowing the intimate interaction between the nickel center and furfuryl alcohol but also enabling the transfer of hydroxide from nickel to the bound furfuryl alcohol.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Roylance JJ, Kim TW, Choi K-S. Efficient and selective electrochemical and photoelectrochemical reduction of 5-hydroxymethylfurfural to 2,5-bis(hydroxymethyl)furan using water as the hydrogen source. ACS Catal. 2016;6:1840–1847. doi: 10.1021/acscatal.5b02586. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

