Enhancing stability by trapping palladium inside N-heterocyclic carbene-functionalized hypercrosslinked polymers for heterogeneous C-C bond formations
- PMID: 33767184
- PMCID: PMC7994585
- DOI: 10.1038/s41467-021-22084-5
Enhancing stability by trapping palladium inside N-heterocyclic carbene-functionalized hypercrosslinked polymers for heterogeneous C-C bond formations
Abstract
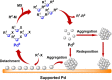
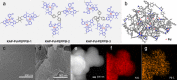
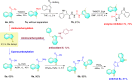
Catalyst deactivation caused by the aggregation of active metal species in the reaction process poses great challenges for practical applications of supported metal catalysts in solid-liquid catalysis. Herein, we develop a hypercrosslinked polymer integrated with N-heterocyclic carbene (NHC) as bifunctional support to stabilize palladium in heterogeneous C-C bond formations. This polymer supported palladium catalyst exhibits excellent stability in the one-pot fluorocarbonylation of indoles to four kinds of valuable indole-derived carbonyl compounds in cascade or sequential manner, as well as the representative Suzuki-Miyaura coupling reaction. Investigations on stabilizing effect disclose that this catalyst displays a molecular fence effect in which the coordination of NHC sites and confinement of polymer skeleton contribute together to stabilize the active palladium species in the reaction process. This work provides new insight into the development of supported metal catalysts with high stability and will also boost their efficient applications in advanced synthesis.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
N-Heterocyclic Carbene-Palladium Polymers Network: Recyclable Pre-catalyst for Effective Suzuki-Miyaura Coupling Reaction in Water.Chem Biodivers. 2023 Jun;20(6):e202201246. doi: 10.1002/cbdv.202201246. Epub 2023 May 30. Chem Biodivers. 2023. PMID: 37186504
-
Recent advances in sustainable N-heterocyclic carbene-Pd(II)-pyridine (PEPPSI) catalysts: A review.Environ Res. 2023 May 15;225:115515. doi: 10.1016/j.envres.2023.115515. Epub 2023 Feb 24. Environ Res. 2023. PMID: 36842701 Review.
-
Palladium-Anchored N-Heterocyclic Carbenes in a Porous Organic Polymer: A Heterogeneous Composite Catalyst for Eco-Friendly C-C Coupling.J Org Chem. 2022 Dec 16;87(24):16655-16664. doi: 10.1021/acs.joc.2c02325. Epub 2022 Nov 25. J Org Chem. 2022. PMID: 36426632
-
A highly active water-soluble cross-coupling catalyst based on dendritic polyglycerol N-heterocyclic carbene palladium complexes.ChemSusChem. 2008;1(7):637-42. doi: 10.1002/cssc.200800042. ChemSusChem. 2008. PMID: 18702166
-
Chelating Bis(N-Heterocyclic Carbene) Palladium-Catalyzed Reactions.Chem Asian J. 2018 Sep 4;13(17):2257-2276. doi: 10.1002/asia.201800583. Epub 2018 Jul 30. Chem Asian J. 2018. PMID: 29863752 Review.
Cited by
-
Hyper crosslinked polymer supported NHC stabilized gold nanoparticles with excellent catalytic performance in flow processes.Nanoscale Adv. 2022 Nov 21;5(4):1095-1101. doi: 10.1039/d2na00799a. eCollection 2023 Feb 14. Nanoscale Adv. 2022. PMID: 36798502 Free PMC article.
-
Porous organic polycarbene nanotrap for efficient and selective gold stripping from electronic waste.Nat Commun. 2023 Jan 17;14(1):263. doi: 10.1038/s41467-023-35971-w. Nat Commun. 2023. PMID: 36650177 Free PMC article.
-
Integrated "all-in-one" strategy to construct highly efficient Pd catalyst for CO2 transformation.Chem Sci. 2024 Aug 27;15(37):15321-31. doi: 10.1039/d4sc03106g. Online ahead of print. Chem Sci. 2024. PMID: 39246380 Free PMC article.
-
Dipolar Microenvironment Engineering Enabled by Electron Beam Irradiation for Boosting Catalytic Performance.Adv Sci (Weinh). 2024 Aug;11(30):e2401562. doi: 10.1002/advs.202401562. Epub 2024 Jun 11. Adv Sci (Weinh). 2024. PMID: 38860673 Free PMC article.
-
Palladium nanoparticles for the synthesis of phenanthridinones and benzo[c]chromenes via C-H activation reaction.RSC Adv. 2024 Jun 11;14(26):18703-18715. doi: 10.1039/d4ra02835j. eCollection 2024 Jun 6. RSC Adv. 2024. PMID: 38863826 Free PMC article.
References
-
- Miyaura N, Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. - DOI
-
- Stille JK. The palladium-catalyzed cross-coupling reactions of organotin reagents with organic electrophiles. Angew. Chem. Int. Ed. 1986;25:508–524. doi: 10.1002/anie.198605081. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

