SARS-CoV-2 infection of the oral cavity and saliva
- PMID: 33767405
- PMCID: PMC8240394
- DOI: 10.1038/s41591-021-01296-8
SARS-CoV-2 infection of the oral cavity and saliva
Abstract
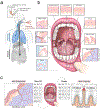
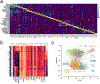
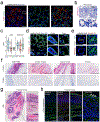
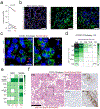
Despite signs of infection-including taste loss, dry mouth and mucosal lesions such as ulcerations, enanthema and macules-the involvement of the oral cavity in coronavirus disease 2019 (COVID-19) is poorly understood. To address this, we generated and analyzed two single-cell RNA sequencing datasets of the human minor salivary glands and gingiva (9 samples, 13,824 cells), identifying 50 cell clusters. Using integrated cell normalization and annotation, we classified 34 unique cell subpopulations between glands and gingiva. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral entry factors such as ACE2 and TMPRSS members were broadly enriched in epithelial cells of the glands and oral mucosae. Using orthogonal RNA and protein expression assessments, we confirmed SARS-CoV-2 infection in the glands and mucosae. Saliva from SARS-CoV-2-infected individuals harbored epithelial cells exhibiting ACE2 and TMPRSS expression and sustained SARS-CoV-2 infection. Acellular and cellular salivary fractions from asymptomatic individuals were found to transmit SARS-CoV-2 ex vivo. Matched nasopharyngeal and saliva samples displayed distinct viral shedding dynamics, and salivary viral burden correlated with COVID-19 symptoms, including taste loss. Upon recovery, this asymptomatic cohort exhibited sustained salivary IgG antibodies against SARS-CoV-2. Collectively, these data show that the oral cavity is an important site for SARS-CoV-2 infection and implicate saliva as a potential route of SARS-CoV-2 transmission.
Conflict of interest statement
Competing interests
Although the authors view each of these as non-competing financial interests, we report that, in the last year, K.M.B. has been a Scientific Advisor at Arcato Laboratories; K.M.B., B.M.W., M.O.F., A.J.K. and S.T. are active members of the Human Cell Atlas; for the past 6 months, M.O.F. has served as a Scientific Advisor for the PolyBio Research Foundation; and, for the last 3 years, S.A.T. has been remunerated for consulting by Roche and Genentech and is a member of Scientific Advisory Boards at Biogen, GlaxoSmithKline and Foresite Labs. All other authors declare no competing interests.
Figures
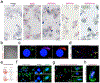





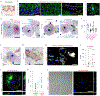

References
-
- Hamner L et al. High SARS-CoV-2 attack rate following exposure at a choir practice—Skagit County, Washington, March 2020. MMWR Morb. Mortal. Wkly. Rep 69, 606–610 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MIKAMI19XX0/Cystic Fibrosis Foundation (CF Foundation)
- NIDDK P30-DK034987/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- Z01 DE000704/ImNIH/Intramural NIH HHS/United States
- L30 DE029055/DE/NIDCR NIH HHS/United States
- T32 GM133364/GM/NIGMS NIH HHS/United States
- KL2 TR002490/TR/NCATS NIH HHS/United States
- NIDCR Z01-DE000704/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- ZIA DE000704/ImNIH/Intramural NIH HHS/United States
- NIDCR K01-DE027087/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- KATO20F0/Cystic Fibrosis Foundation (CF Foundation)
- P30 DK034987/DK/NIDDK NIH HHS/United States
- K01 DE027087/DE/NIDCR NIH HHS/United States
- K08-DE026537 (Byrd)/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- K08 DE026537/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

