Haemovigilance Programme of India: Comparative analysis of transfusion reactions reported over a 5-year period through two reporting formats and key recommendations for blood safety
- PMID: 33767535
- PMCID: PMC7983136
- DOI: 10.4103/ajts.ajts_192_20
Haemovigilance Programme of India: Comparative analysis of transfusion reactions reported over a 5-year period through two reporting formats and key recommendations for blood safety
Conflict of interest statement
There are no conflicts of interest.
Figures
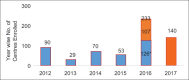
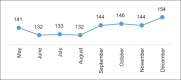


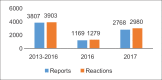
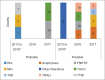
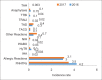


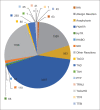
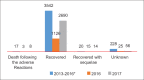

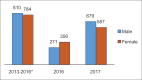
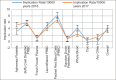




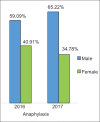
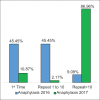
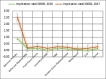

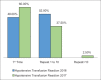
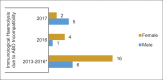

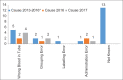



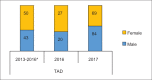
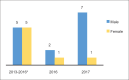
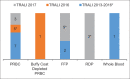
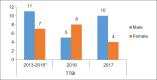
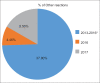
References
-
- Marwaha N, Singh S, Bisht A. Setting up haemovigilance from the very first step.The Indian perspective. Voxs. 2014;9:178–83.
-
- Shivgunde P, Besekar S, Bhojwani K, Bhojwani D. Knowledge, attitude and practice of haemovigilance amongst healthcare professionals in Nashik, Maharashtra, India. IJBCP. 2018;7:986–91.
-
- International Society of Blood Transfusiion Working Party on Haemovigilance. Proposed Standard Definitions for Surveillance of Non-Infectious Adverse Transfusion Reactions. 2011. [Last accessed on 2020 Oct 16]. Available from: http//www.isbtweb.org/Haemovigilance/ISBT_definitions_final_2011 .
LinkOut - more resources
Full Text Sources
