Spontaneous Afferent Activity Carves Olfactory Circuits
- PMID: 33767612
- PMCID: PMC7985084
- DOI: 10.3389/fncel.2021.637536
Spontaneous Afferent Activity Carves Olfactory Circuits
Abstract
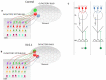
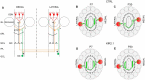
Electrical activity has a key role in shaping neuronal circuits during development. In most sensory modalities, early in development, internally generated spontaneous activity sculpts the initial layout of neuronal wiring. With the maturation of the sense organs, the system relies more on sensory-evoked electrical activity. Stimuli-driven neuronal discharge is required for the transformation of immature circuits in the specific patterns of neuronal connectivity that subserve normal brain function. The olfactory system (OS) differs from this organizational plan. Despite the important role of odorant receptors (ORs) in shaping olfactory topography, odor-evoked activity does not have a prominent role in refining neuronal wiring. On the contrary, afferent spontaneous discharge is required to achieve and maintain the specific diagram of connectivity that defines the topography of the olfactory bulb (OB). Here, we provide an overview of the development of olfactory topography, with a focus on the role of afferent spontaneous discharge in the formation and maintenance of the specific synaptic contacts that result in the topographic organization of the OB.
Keywords: activity; development; olfaction; plasticity; spontaneous; topography.
Copyright © 2021 Redolfi and Lodovichi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Circuit formation and function in the olfactory bulb of mice with reduced spontaneous afferent activity.J Neurosci. 2015 Jan 7;35(1):146-60. doi: 10.1523/JNEUROSCI.0613-14.2015. J Neurosci. 2015. PMID: 25568110 Free PMC article.
-
GABAB Receptors Tune Cortical Feedback to the Olfactory Bulb.J Neurosci. 2016 Aug 10;36(32):8289-304. doi: 10.1523/JNEUROSCI.3823-15.2016. J Neurosci. 2016. PMID: 27511004 Free PMC article.
-
Effect of Interglomerular Inhibitory Networks on Olfactory Bulb Odor Representations.J Neurosci. 2020 Jul 29;40(31):5954-5969. doi: 10.1523/JNEUROSCI.0233-20.2020. Epub 2020 Jun 19. J Neurosci. 2020. PMID: 32561671 Free PMC article.
-
Odorant receptors signaling instructs the development and plasticity of the glomerular map.Neural Plast. 2015;2015:975367. doi: 10.1155/2015/975367. Epub 2015 Jan 20. Neural Plast. 2015. PMID: 25688305 Free PMC article. Review.
-
Developmental regulation of olfactory circuit formation in mice.Dev Growth Differ. 2020 May;62(4):199-213. doi: 10.1111/dgd.12657. Epub 2020 Feb 28. Dev Growth Differ. 2020. PMID: 32112394 Free PMC article. Review.
Cited by
-
The role of the odorant receptors in the formation of the sensory map.BMC Biol. 2021 Aug 27;19(1):174. doi: 10.1186/s12915-021-01116-y. BMC Biol. 2021. PMID: 34452614 Free PMC article. Review.
-
Development of the mammalian main olfactory bulb.Development. 2022 Feb 1;149(3):dev200210. doi: 10.1242/dev.200210. Epub 2022 Feb 11. Development. 2022. PMID: 35147186 Free PMC article. Review.
-
The frontal sharp transient in newborns: An endogenous neurobiomarker concomitant to the physiological and critical transitional period around delivery?Cereb Cortex. 2023 Mar 21;33(7):4026-4039. doi: 10.1093/cercor/bhac324. Cereb Cortex. 2023. PMID: 36066405 Free PMC article.
-
Epigenetic and Transcriptional Regulation of Spontaneous and Sensory Activity Dependent Programs During Neuronal Circuit Development.Front Neural Circuits. 2022 May 18;16:911023. doi: 10.3389/fncir.2022.911023. eCollection 2022. Front Neural Circuits. 2022. PMID: 35664458 Free PMC article. Review.
-
SARS-CoV-2 Brain Regional Detection, Histopathology, Gene Expression, and Immunomodulatory Changes in Decedents with COVID-19.J Neuropathol Exp Neurol. 2022 Aug 16;81(9):666-695. doi: 10.1093/jnen/nlac056. J Neuropathol Exp Neurol. 2022. PMID: 35818336 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

