An Overview of FGF-23 as a Novel Candidate Biomarker of Cardiovascular Risk
- PMID: 33767635
- PMCID: PMC7985069
- DOI: 10.3389/fphys.2021.632260
An Overview of FGF-23 as a Novel Candidate Biomarker of Cardiovascular Risk
Abstract
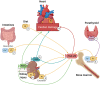
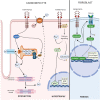
Fibroblast growth factor-23 (FGF)-23 is a phosphaturic hormone involved in mineral bone metabolism that helps control phosphate homeostasis and reduces 1,25-dihydroxyvitamin D synthesis. Recent data have highlighted the relevant direct FGF-23 effects on the myocardium, and high plasma levels of FGF-23 have been associated with adverse cardiovascular outcomes in humans, such as heart failure and arrhythmias. Therefore, FGF-23 has emerged as a novel biomarker of cardiovascular risk in the last decade. Indeed, experimental data suggest FGF-23 as a direct mediator of cardiac hypertrophy development, cardiac fibrosis and cardiac dysfunction via specific myocardial FGF receptor (FGFR) activation. Therefore, the FGF-23/FGFR pathway might be a suitable therapeutic target for reducing the deleterious effects of FGF-23 on the cardiovascular system. More research is needed to fully understand the intracellular FGF-23-dependent mechanisms, clarify the downstream pathways and identify which could be the most appropriate targets for better therapeutic intervention. This review updates the current knowledge on both clinical and experimental studies and highlights the evidence linking FGF-23 to cardiovascular events. The aim of this review is to establish the specific role of FGF-23 in the heart, its detrimental effects on cardiac tissue and the possible new therapeutic opportunities to block these effects.
Keywords: FGF-23; FGFR; LVH; heart; heart failure – pharmacological treatment – systolic dysfunction.
Copyright © 2021 Vázquez-Sánchez, Poveda, Navarro-García, González-Lafuente, Rodríguez-Sánchez, Ruilope and Ruiz-Hurtado.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Alvarado F. J., Chen X., Valdivia H. H. (2017). Ablation of the cardiac ryanodine receptor phospho-site Ser2808 does not alter the adrenergic response or the progression to heart failure in mice. Elimination of the genetic background as critical variable. J. Mol. Cell Cardiol. 103 40–47. 10.1016/j.yjmcc.2017.01.001 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

