Optical Coherence Tomography Angiography for the Evaluation of Retinal Vasculature in Fabry Disease: Our Experience and Review of Current Knowledge
- PMID: 33767663
- PMCID: PMC7985262
- DOI: 10.3389/fneur.2021.640719
Optical Coherence Tomography Angiography for the Evaluation of Retinal Vasculature in Fabry Disease: Our Experience and Review of Current Knowledge
Abstract
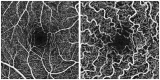
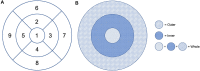
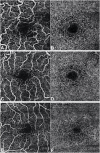
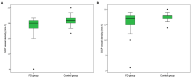
Purpose: Optical coherence tomography angiography (OCTA) is a non-invasive and objective tool for the evaluation of the retinal microvascular changes in Fabry disease (FD). We investigated changes in retinal vasculature in FD patients, and the possible correlation with systemic parameters, by using OCTA, and reviewed the current status of literature. Methods: Thirteen FD patients (eight females, five males, mean age 49.85 ± 14.7 years) were compared with 13 age- and sex-matched healthy controls. OCTA 3 × 3 mm macular scans were performed in all subjects. We evaluated the vessel density and vessel perfusion in distinct macular areas (whole, inner, and outer) of both the superficial capillary plexus (SCP VD and SCP VP) and of the deep capillary plexus (DCP VD and DCP VP). We also evaluated the foveal avascular zone (FAZ) metrics (area, perimeter, and circularity), and correlation between systemic and OCTA parameters. A literature review on the current understanding of OCTA in FD is then presented. Results: FD patients showed significantly lower SCP VD values in the whole area (17.37 ± 2.08 mm-1 vs. 18.54 ± 1.21 mm-1; p-value 0.022), as well as in the outer area (17.46 ± 2.10 mm-1 vs. 19.08 ± 1.14 mm-1; p-value 0.002), but not in the inner. Even the DCP VD was significantly lower in all the imaged areas: whole (17.75 ± 3.93 mm-1 vs. 19.71 ± 1.20 mm-1; p-value 0.024), outer (18.25 ± 4.17 mm-1 vs. 20.33 ± 1.20 mm-1; p-value 0.023), and inner (19.54 ± 4.17 mm-1 vs. 21.96 ± 1.55 mm-1; p-value 0.011). There were no significant differences in vessel perfusion parameters (both SCP VP and DCP VP ones) and FAZ. No significant correlations were found between the OCTA parameters and systemic parameters (maximal left ventricular wall thickness and glomerular filtration rate) in FD patients. Conclusions: OCTA can be considered as a promising non-invasive tool, which enables a quantitative evaluation of retinal vascular involvement in FD, despite the varying data reported in literature. Our results support the use of OCTA as an objective tool to evaluate retinal vascular abnormalities in FD. The utility of OCTA in FD needs to be validated by longitudinal studies taking into account the overall progression of the disease.
Keywords: Fabry disease; OCTA; optical coherenc tomography angiography; vascular density; vascular perfusion.
Copyright © 2021 Bacherini, Vicini, Nicolosi, Tanini, Lenzetti, Finocchio, Cirami, Dervishi, Rizzo, Virgili, Giansanti and Sodi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

