Molecular Characteristics, Antigenicity, Pathogenicity, and Zoonotic Potential of a H3N2 Canine Influenza Virus Currently Circulating in South China
- PMID: 33767679
- PMCID: PMC7985081
- DOI: 10.3389/fmicb.2021.628979
Molecular Characteristics, Antigenicity, Pathogenicity, and Zoonotic Potential of a H3N2 Canine Influenza Virus Currently Circulating in South China
Abstract
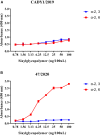
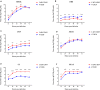
Canine influenza viruses (CIVs) could be a source of influenza viruses which infect humans because canine are important companion pets. To assess the potential risk of H3N2 CIVs currently circulating in southern China to public health, biological characteristics of A/canine/Guangdong/DY1/2019 (CADY1/2019) were detected. CADY1/2019 bound to both avian-type and human-type receptors. CADY1/2019 had a similar pH value for HA protein fusion to human viruses, but its antigenicity was obviously different from those of current human H3N2 influenza viruses (IVs) or the vaccine strains recommended in the North hemisphere. CADY1/2019 effectively replicated in the respiratory tract and was transmitted by physical contact among guinea pigs. Compared to human H3N2 IV, CADY1/2019 exhibited higher replication in MDCK, A549, 3D4/21, ST, and PK15 cells. Sequence analysis indicated that CADY1/2019 is an avian-origin virus, and belongs to the novel clade and has acquired many adaptation mutations to infect other mammals, including human. Taken together, currently circulating H3N2 CIVs have a zoonotic potential, and there is a need for strengthening surveillance and monitoring of their pathogenicity.
Keywords: H3N2 canine influenza virus; HA stability; antigenicity; guinea pig; pathogenicity.
Copyright © 2021 Wu, Su, Gu, Yu, Li, Sun, Pan, Cui, Zhu, Yang, Liu, Xu, Li, Liu, Qu, Wu, Liao and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Alexander D. J., Brown I. H. (2000). Recent zoonoses caused by influenza a viruses. Rev. Sci. Tech. 19 197–225. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

