The BCMA-Targeted Fourth-Generation CAR-T Cells Secreting IL-7 and CCL19 for Therapy of Refractory/Recurrent Multiple Myeloma
- PMID: 33767695
- PMCID: PMC7985831
- DOI: 10.3389/fimmu.2021.609421
The BCMA-Targeted Fourth-Generation CAR-T Cells Secreting IL-7 and CCL19 for Therapy of Refractory/Recurrent Multiple Myeloma
Abstract
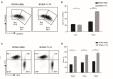
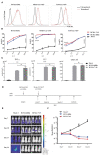
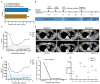
Chimeric antigen receptor (CAR) technology has revolutionized cancer treatment, particularly in malignant hematological tumors. Currently, the BCMA-targeted second-generation CAR-T cells have showed impressive efficacy in the treatment of refractory/relapsed multiple myeloma (R/R MM), but up to 50% relapse remains to be addressed urgently. Here we constructed the BCMA-targeted fourth-generation CAR-T cells expressing IL-7 and CCL19 (i.e., BCMA-7 × 19 CAR-T cells), and demonstrated that BCMA-7 × 19 CAR-T cells exhibited superior expansion, differentiation, migration and cytotoxicity. Furthermore, we have been carrying out the first-in-human clinical trial for therapy of R/R MM by use of BCMA-7 × 19 CAR-T cells (ClinicalTrials.gov Identifier: NCT03778346), which preliminarily showed promising safety and efficacy in first two enrolled patients. The two patients achieved a CR and VGPR with Grade 1 cytokine release syndrome only 1 month after one dose of CAR-T cell infusion, and the responses lasted more than 12-month. Taken together, BCMA-7 × 19 CAR-T cells were safe and effective against refractory/relapsed multiple myeloma and thus warranted further clinical study.
Keywords: BCMA; CAR-T; CCL19; IL-7; multiple myeloma.
Copyright © 2021 Duan, Wang, Wei, Feng, Liu, He, Xu, Wang, Zhao, Lv, Long, Lin, Zhao, Fang, Jiang, Tang and Gao.
Conflict of interest statement
AZ and JG were employed by Zhejiang Qixin Biotech. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Efficacy of Humanized Anti-BCMA CAR T Cell Therapy in Relapsed/Refractory Multiple Myeloma Patients With and Without Extramedullary Disease.Front Immunol. 2021 Aug 5;12:720571. doi: 10.3389/fimmu.2021.720571. eCollection 2021. Front Immunol. 2021. PMID: 34421924 Free PMC article.
-
A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma.J Hematol Oncol. 2021 Oct 9;14(1):161. doi: 10.1186/s13045-021-01170-7. J Hematol Oncol. 2021. PMID: 34627333 Free PMC article. Clinical Trial.
-
B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma.Clin Cancer Res. 2013 Apr 15;19(8):2048-60. doi: 10.1158/1078-0432.CCR-12-2422. Epub 2013 Jan 23. Clin Cancer Res. 2013. PMID: 23344265 Free PMC article.
-
Efficacy and safety of chimeric antigen receptor T cells targeting BCMA and GPRC5D in relapsed or refractory multiple myeloma.Front Immunol. 2024 Dec 23;15:1466443. doi: 10.3389/fimmu.2024.1466443. eCollection 2024. Front Immunol. 2024. PMID: 39763668 Free PMC article.
-
Mechanisms and salvage treatments in patients with multiple myeloma relapsed post-BCMA CAR-T cell therapy.Front Immunol. 2024 Oct 22;15:1433774. doi: 10.3389/fimmu.2024.1433774. eCollection 2024. Front Immunol. 2024. PMID: 39502704 Free PMC article. Review.
Cited by
-
Exploring CAR-T Cell Therapy Side Effects: Mechanisms and Management Strategies.J Clin Med. 2023 Sep 22;12(19):6124. doi: 10.3390/jcm12196124. J Clin Med. 2023. PMID: 37834768 Free PMC article. Review.
-
CSF1R Inhibition Combined with GM-CSF Reprograms Macrophages and Disrupts Protumoral Interplays with AML Cells.Cancers (Basel). 2021 Oct 21;13(21):5289. doi: 10.3390/cancers13215289. Cancers (Basel). 2021. PMID: 34771453 Free PMC article.
-
CAR T therapies in multiple myeloma: unleashing the future.Cancer Gene Ther. 2024 May;31(5):667-686. doi: 10.1038/s41417-024-00750-2. Epub 2024 Mar 4. Cancer Gene Ther. 2024. PMID: 38438559 Free PMC article. Review.
-
CAR-T Therapy in Relapsed Refractory Multiple Myeloma.Curr Med Chem. 2024;31(27):4362-4382. doi: 10.2174/0109298673268932230920063933. Curr Med Chem. 2024. PMID: 37779413 Free PMC article. Review.
-
Payload Delivery: Engineering Immune Cells to Disrupt the Tumour Microenvironment.Cancers (Basel). 2021 Nov 29;13(23):6000. doi: 10.3390/cancers13236000. Cancers (Basel). 2021. PMID: 34885108 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

