PD-1 Blockade Modulates Functional Activities of Exhausted-Like T Cell in Patients With Cutaneous Leishmaniasis
- PMID: 33767700
- PMCID: PMC7985249
- DOI: 10.3389/fimmu.2021.632667
PD-1 Blockade Modulates Functional Activities of Exhausted-Like T Cell in Patients With Cutaneous Leishmaniasis
Abstract
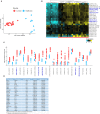
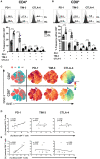
Patients infected by Leishmania braziliensis develop debilitating skin lesions. The role of inhibitory checkpoint receptors (ICRs) that induce T cell exhaustion during this disease is not known. Transcriptional profiling identified increased expression of ICRs including PD-1, PDL-1, PDL-2, TIM-3, and CTLA-4 in skin lesions of patients that was confirmed by immunohistology where there was increased expression of PD-1, TIM-3, and CTLA-4 in both CD4+ and CD8+ T cell subsets. Moreover, PDL-1/PDL-2 ligands were increased on skin macrophages compared to healthy controls. The proportions PD1+, but not TIM-3 or CTLA-4 expressing T cells in the circulation were positively correlated with those in the lesions of the same patients, suggesting that PD-1 may regulate T cell function equally in both compartments. Blocking PD-1 signaling in circulating T cells enhanced their proliferative capacity and IFN-γ production, but not TNF-α secretion in response to L. braziliensis recall antigen challenge in vitro. While we previously showed a significant correlation between the accumulation of senescent CD8+CD45RA+CD27- T cells in the circulation and skin lesion size in the patients, there was no such correlation between the extent of PD-1 expression by circulating on T cells and the magnitude of skin lesions suggesting that exhausted-like T cells may not contribute to the cutaneous immunopathology. Nevertheless, we identified exhausted-like T cells in both skin lesions and in the blood. Targeting this population by PD-1 blockade may improve T cell function and thus accelerate parasite clearance that would reduce the cutaneous pathology in cutaneous leishmaniasis.
Keywords: Leishmania braziliensis; PD-1; T cell exhaustion; cutaneous leishmaniasis; immunosenescence; inhibitory checkpoint receptors; senescent T cells.
Copyright © 2021 Garcia de Moura, Covre, Fantecelle, Gajardo, Cunha, Stringari, Belew, Daniel, Zeidler, Tadokoro, de Matos Guedes, Zanotti, Mosser, Falqueto, Akbar and Gomes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a shared affiliation, though no other collaboration, with one of the authors HM.
Figures



References
-
- World Health Organization . OMS | Leishmaniasis. In: WHO, vol. 375. (2015). Available at: http://whqlibdoc.who.int/trs/WHO_TRS_949_eng.pdf.
-
- Ribeiro-Romao RP, Moreira OC, Osorio EY, Cysne-Finkelstein L, Gomes-Silva A, Valverde JG, et al. Comparative Evaluation of Lesion Development, Tissue Damage, and Cytokine Expression in Golden Hamsters (Mesocricetus auratus) Infected by Inocula with Different Leishmania (Viannia) braziliensis Concentrations. Infect Immun (2014) 82:5203–13. 10.1128/IAI.02083-14 - DOI - PMC - PubMed
-
- Costa JML, Saldanha ACR, Nasciento D, Sampaio G, Carneiro F, Lisboa E, et al. Clinical modalities, diagnosis and therapeutic approach of tegumentary leishmaniasis in Brazil. Gaz Med Da Bahia (2009) 143:70–83.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

