Blood Interferon-α Levels and Severity, Outcomes, and Inflammatory Profiles in Hospitalized COVID-19 Patients
- PMID: 33767713
- PMCID: PMC7985458
- DOI: 10.3389/fimmu.2021.648004
Blood Interferon-α Levels and Severity, Outcomes, and Inflammatory Profiles in Hospitalized COVID-19 Patients
Abstract
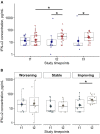
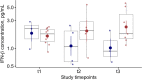
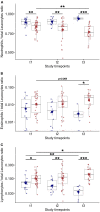
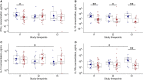
Background: Deficient interferon responses have been proposed as one of the relevant mechanisms prompting severe manifestations of COVID-19. Objective: To evaluate the interferon (IFN)-α levels in a cohort of COVID-19 patients in relation to severity, evolution of the clinical manifestations and immune/inflammatory profile. Methods: This is prospective study recruiting consecutive hospitalized patients with respiratory failure associated with SARS-COV-2 infection and matched controls. After enrollment, patients were assessed every 7 ± 2 days for additional 2 consecutive visits, for a total of 21 days. The severity of the clinical condition was ranked based on the level of respiratory support required. At each time-point blood samples were obtained to assess immune cells and mediators by multiplex immunoassay. Results: Fifty-four COVD-19 and 11 control patients matched for severity were enrolled. At recruitment, lower levels of blood IFN-α were found in COVID-19 patients compared to controls (3.8-fold difference, p < 0.01). Improvements in COVID-19 severity were paralleled by a significant increase of blood IFN-α levels. A significant increase in blood IFN-α was found over the study period in survivors (70% of the study population). A similar trend was found for blood IFN-β with IFN-β levels below the threshold of detectability in a substantial proportion of subjects. Significantly higher values of blood lymphocytes and lower levels of IL-10 were found at each time point in patients who survived compared to patients who died. In patients who clinically improved and survived during the study, we found an inverse association between IL-10 and IFN-α levels. Conclusion: The study identifies a blood immune profile defined by deficient IFN-α levels associated with increased IL-10 expression in patients progressing to severe/life threatening COVID-19 conditions, suggesting the involvement of immunological pathways that could be target of pharmacological intervention. Clinical Trial Registration: ClinicalTrials.gov identifier NCT04343053.
Keywords: COVID−19; SARS–CoV−2; interferon; mortality; respiratory failure.
Copyright © 2021 Contoli, Papi, Tomassetti, Rizzo, Vieceli Dalla Sega, Fortini, Torsani, Morandi, Ronzoni, Zucchetti, Pavasini, Fogagnolo, Volta, Bartlett, Johnston, Spadaro and Campo.
Conflict of interest statement
MC reports grants, personal fees and non-financial support from Chiesi, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, personal fees and non-financial support from Alk-Abello, grants, personal fees and non-financial support from GlaxoSmithKline, personal fees and non-financial support from Novartis, personal fees and non-financial support from Zambon, grants from University of Ferrara - Italy, outside the submitted work. AP reports grants, personal fees, non-financial support and other from GlaxoSmithKline, grants, personal fees and non-financial support from AstraZeneca, grants, personal fees, non-financial support and other from Boehringer Ingelheim, grants, personal fees, non-financial support and other from Chiesi Farmaceutici, grants, personal fees, non-financial support and other from TEVA, personal fees, non-financial support and other from Mundipharma, personal fees, non-financial support and other from Zambon, personal fees, non-financial support and other from Novartis, grants, personal fees and non-financial support from Menarini, personal fees, non-financial support and other from Sanofi/Regeneron, personal fees from Roche, grants from Fondazione Maugeri, grants from Fondazione Chiesi, personal fees from Edmondpharma, outside the submitted work. SJ reports personal fees from Virtus Respiratory Research, personal fees from Myelo Therapeutics GmbH, personal fees from Concert Pharmaceuticals, personal fees from Bayer, personal fees from Synairgen, personal fees from Novartis, personal fees from Boehringer Ingelheim, personal fees from Chiesi, personal fees from Gerson Lehrman Group, personal fees from resTORbio, personal fees from Bioforce, personal fees from Materia Medical Holdings, personal fees from PrepBio Pharma, personal fees from Pulmotect, personal fees from Virion Health, personal fees from Lallemand Pharma, personal fees from AstraZeneca, outside the submitted work and has a patent Wark PA, Johnston SL, Holgate ST, Davies DE. Anti-virus therapy for respiratory diseases. UK patent application No. GB 0405634.7, 12 March 2004. with royalties paid, a patent Wark PA, Johnston SL, Holgate ST, Davies DE. Interferon-Beta for Anti-Virus Therapy for Respiratory Diseases. International Patent Application No. PCT/ GB05/50031, 12 March 2004. with royalties paid, and a patent Davies DE, Wark PA, Holgate ST, Johnston SL. Interferon Lambda therapy for the treatment of respiratory disease. UK patent application No. 6779645.9, granted 15th August 2012. licensed. GC reports grants and personal fees from Astrazeneca, grants and personal fees from Boston Scientific, grants and personal fees from SMT, grants and personal fees from Eukon, grants and personal fees from Daiichi Sankyo, grants and personal fees from Menarini, outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

