GLP-1 receptor agonists: an updated review of head-to-head clinical studies
- PMID: 33767808
- PMCID: PMC7953228
- DOI: 10.1177/2042018821997320
GLP-1 receptor agonists: an updated review of head-to-head clinical studies
Abstract
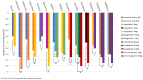
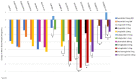
Glucagon-like peptide-1 receptor agonists (GLP-1 RA) are attractive options for the treatment of type 2 diabetes (T2D) because they effectively lower A1C and weight while having a low risk of hypoglycemia. Some also have documented cardiovascular benefit. The GLP-1 RA class has grown in the last decade, with several agents available for use in the United States and Europe. Since the efficacy and tolerability, dosing frequency, administration requirements, and cost may vary between agents within the class, each agent may offer unique advantages and disadvantages. Through a review of phase III clinical trials studying dulaglutide, exenatide twice daily, exenatide once weekly, liraglutide, lixisenatide, semaglutide, and oral semaglutide, 14 head-to-head trials were identified that evaluated the safety and efficacy of GLP-1 RA active comparators. The purpose of this review is to provide an analysis of these trials. The GLP-1 RA head-to-head clinical studies have demonstrated that all GLP-1 RA agents are effective therapeutic options at reducing A1C. However, differences exist in terms of magnitude of effect on A1C and weight as well as frequency of adverse effects.
Keywords: GLP-1 receptor agonist; type 2 diabetes.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: JT (Sanofi, advisory board member)
Figures
References
-
- International Diabetes Federation. IDF diabetes atlas. 9th ed. Brussels, Belgium: IDF, 2019. https://www.diabetesatlas.org (accessed 1 July 2020).
-
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment. Standards of Medical Care in Diabetes – 2021. Diabetes Care 2021; 44(Suppl. 1): S111–S124. - PubMed
-
- Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of clinical endocrinologists and American College of endocrinology on the comprehensive type 2 diabetes management algorithm – 2020 executive summary. Endocr Pract 2020; 26: 107–139. - PubMed
-
- Trujillo JM. Glucagon-like peptide-1 receptor agonists. In: White JR. (ed.) Guide to medications for the treatment of diabetes mellitus. Arlington County, VA: American Diabetes Association, 2020, pp.190–210.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

