Neuroendocrine small cell carcinoma of the cervix: A case report
- PMID: 33767861
- PMCID: PMC7976432
- DOI: 10.3892/mco.2021.2254
Neuroendocrine small cell carcinoma of the cervix: A case report
Abstract
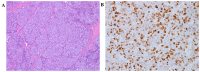
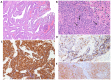
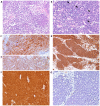
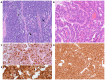
Merkel cell polyomavirus (MCPyV) has been found in patients with Merkel cell carcinoma and respiratory tract infections. Merkel cell carcinoma is a primary aggressive neuroendocrine carcinoma of the skin. It has been demonstrated that MCPyV can be transmitted during sexual activity and may be present in the oral and anogenital mucosa. The aim of the present study was to evaluate whether MCPyV coexisted with HPV in three cases of neuroendocrine small cell carcinoma of the cervix using PCR and immunohistochemical analysis Three cases of NSC of the cervix were identified in the pathology archives of Parma University (Italy). Of these, two cases were associated with an adenocarcinomatous component. A set of general primers from the L1 region (forward, L1C1 and reverse, L1C2 or L1C2M) was PCR amplified to detect the broad-spectrum DNA of genital HPV. The presence of MCPyV was investigated via immunohistochemistry using a mouse monoclonal antibody against the MCPyV LT antigen and through PCR analysis to separate viral DNA. HPV DNA was present in all three neuroendocrine carcinomas and in the adenocarcinoma component of the two mixed cases. None of the cases were immunoreactive to CM2B4 and did not contain viral DNA in either their neuroendocrine or adenocarcinomatous component. Whilst it is difficult to draw definitive conclusions from such a small sample size, these data suggested that MCPyV does not coexist with HPV in the cervix. However, in the present study, the absence of detectable MCPyV may have been due to the presence of a genotype that was not detected by the primers used in the PCR analysis or by the antibody used for the immunohistochemical study. MCPyV microRNA may also have been present, inhibiting LT expression. Additional studies with larger cohorts and more advanced molecular biology techniques are required to confirm the hypothesis of the current study.
Keywords: human papilloma virus; immunohistochemistry; merkel cell polyomavirus; neuroendocrine small cell carcinoma; polymerase chain reaction.
Copyright: © Giordano et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
A monoclonal antibody against SV40 large T antigen (PAb416) does not label Merkel cell carcinoma.Histopathology. 2018 Jul;73(1):162-166. doi: 10.1111/his.13483. Epub 2018 Apr 19. Histopathology. 2018. PMID: 29430700 Free PMC article.
-
Detection of Merkel cell polyomavirus in Merkel cell carcinomas and small cell carcinomas by PCR and immunohistochemistry.Histol Histopathol. 2011 Oct;26(10):1231-41. doi: 10.14670/HH-26.1231. Histol Histopathol. 2011. PMID: 21870327
-
Merkel cell polyomavirus: a specific marker for Merkel cell carcinoma in histologically similar tumors.Am J Surg Pathol. 2009 Dec;33(12):1771-7. doi: 10.1097/PAS.0b013e3181ba7b73. Am J Surg Pathol. 2009. PMID: 19809278
-
Merkel cell polyomavirus and non-Merkel cell carcinomas: guilty or circumstantial evidence?APMIS. 2020 Feb;128(2):104-120. doi: 10.1111/apm.13019. Epub 2020 Jan 28. APMIS. 2020. PMID: 31990105 Review.
-
Human Merkel cell polyomavirus: virological background and clinical implications.APMIS. 2013 Aug;121(8):755-69. doi: 10.1111/apm.12122. Epub 2013 Jun 19. APMIS. 2013. PMID: 23781869 Review.
Cited by
-
Cytological features of small cell neuroendocrine carcinoma of the uterine cervix.Oncol Lett. 2025 Jun 18;30(2):401. doi: 10.3892/ol.2025.15147. eCollection 2025 Aug. Oncol Lett. 2025. PMID: 40606303 Free PMC article.
-
A Rare Case of Small-Cell Neuroendocrine Cancer of the Cervix: An Unexpected Diagnosis.Cureus. 2023 Jan 20;15(1):e34006. doi: 10.7759/cureus.34006. eCollection 2023 Jan. Cureus. 2023. PMID: 36811044 Free PMC article.
References
-
- Albores-Saavedra J, Gersell D, Gilk CB, Henson DE, Lindberg G, Santiago H, Scully RE, Silva E, Sobin LH, Tavassoli FJ, et al. Terminology of endocrine tumors of the uterine cervix: Results of a work-shop sponsored by College of American Pathologists and the National Cancer Institute. Arch Lab. 1997;121:34–39. - PubMed
-
- Cree IA, Malpica A, McCluggage WG. Neuroendocrine neoplasia in WHO classification of femal genital Tumours 5th edition. Lyon, IARC, pp452-458, 2019.
-
- Ambros RA, Park JS, Shah KV, Kurman RJ. Evaluation of histologic, morphometric, and immunohistochemical criteria in the differential diagnosis of small cell carcinomas of the cervix with particular reference to human papillomavirus types 16 and 18. Mod Pathol. 1991;4:586–593. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
