Useful Parameters in Dynamic Contrast-enhanced Ultrasonography for Identifying Early Response to Chemotherapy in a Rat Liver Tumor Model
- PMID: 33767907
- PMCID: PMC7981939
- DOI: 10.25259/JCIS_6_2020
Useful Parameters in Dynamic Contrast-enhanced Ultrasonography for Identifying Early Response to Chemotherapy in a Rat Liver Tumor Model
Abstract
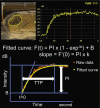
Objectives: The objective of the study is to determine a parameter on the time-intensity curve (TIC) of dynamic contrast-enhanced ultrasonography (DCE-US) that best correlates with tumor growth and to evaluate whether the parameter could correlate with the early response to irinotecan in a rat liver tumor model.
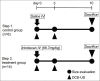
Material and methods: Twenty rats with tumors were evaluated (control: Saline, n = 6; treatment: Irinotecan, n = 14) regarding four parameters from TIC: Peak intensity (PI), k value, slope (PI × k), and time to peak (TTP). Relative changes in maximum tumor diameter between day 0 and 10, and parameters in the first 3 days were evaluated. The Mann-Whitney U-test was used to compare differences in tumor size and other parameters. Pearson's correlation coefficients (r) between tumor size and parameters in the control group were calculated. In the treatment group, relative changes of parameters in the first 3 days were compared between responder and non-responder (<20% and ≥20% increase in size on day 10, respectively).
Results: PI, k value, PI × k, and TTP significantly correlated with tumor growth (r = 0.513, 0.911, 0.665, and 0.741, respectively). The mean RC in k value among responders (n = 6) was significantly lower than non-responders (n = 8) (mean k value, 4.96 vs. 72.5; P = 0.003).
Conclusion: Parameters of DCE-US could be a useful parameter for identifying early response to irinotecan.
Keywords: Biomarkers; Colon cancer; Contrast media; Irinotecan; Rats; Ultrasonography.
© 2021 Published by Scientific Scholar on behalf of Journal of Clinical Imaging Science.
Conflict of interest statement
There are no conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
