Activation of AMPK Promotes Maturation of Cardiomyocytes Derived From Human Induced Pluripotent Stem Cells
- PMID: 33768096
- PMCID: PMC7985185
- DOI: 10.3389/fcell.2021.644667
Activation of AMPK Promotes Maturation of Cardiomyocytes Derived From Human Induced Pluripotent Stem Cells
Abstract
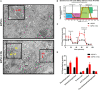
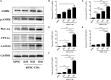
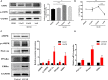
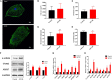
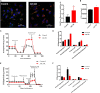
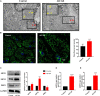
Human induced pluripotent stem cell (iPSC)-derived cardiomyocytes (CMs) (hiPSC-CMs) are a promising cell source for disease modeling, myocardial regeneration, and drug assessment. However, hiPSC-CMs have certain immature fetal CM-like properties that are different from the characteristics of adult CMs in several aspects, including cellular structure, mitochondrial function, and metabolism, thus limiting their applications. Adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK) is an energy-sensing protein kinase involved in the regulation of fatty acid oxidation and mitochondrial biogenesis in cardiomyocytes. This study investigated the effects of AMPK on the maturation of hiPSC-CMs. Activation of AMPK in hiPSC-CMs significantly increased the expression of CM-specific markers and resulted in a more mature myocardial structure compared to that in the control cells. We found that activation of AMPK improved mitochondrial oxidative phosphorylation (OxPhos) and the oxygen consumption rate (OCR). Additionally, our data demonstrated that activation of AMPK increased mitochondrial fusion to promote the maturation of mitochondrial structure and function. Overall, activation of AMPK is an effective approach to promote hiPSC-CMs maturation, which may enhance the utility of hiPSC-CMs in clinical applications.
Keywords: AMPK; cardiomyocyte maturation; hiPSC-CMs; hiPSCs; mitochondrial.
Copyright © 2021 Ye, Zhang, Zhou, Tan, Xu, Yi, Yan, Xie, Zhang, Tian and Zhu.
Figures

Similar articles
-
AMPK activator-treated human cardiac spheres enhance maturation and enable pathological modeling.Stem Cell Res Ther. 2023 Nov 8;14(1):322. doi: 10.1186/s13287-023-03554-7. Stem Cell Res Ther. 2023. PMID: 37941041 Free PMC article.
-
Fatty Acid-Treated Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes Exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes.Cells. 2019 Sep 17;8(9):1095. doi: 10.3390/cells8091095. Cells. 2019. PMID: 31533262 Free PMC article.
-
Targeting HIF-1α in combination with PPARα activation and postnatal factors promotes the metabolic maturation of human induced pluripotent stem cell-derived cardiomyocytes.J Mol Cell Cardiol. 2019 Jul;132:120-135. doi: 10.1016/j.yjmcc.2019.05.003. Epub 2019 May 11. J Mol Cell Cardiol. 2019. PMID: 31082397 Free PMC article.
-
Maturation of pluripotent stem cell-derived cardiomyocytes: limitations and challenges from metabolic aspects.Stem Cell Res Ther. 2024 Oct 8;15(1):354. doi: 10.1186/s13287-024-03961-4. Stem Cell Res Ther. 2024. PMID: 39380099 Free PMC article. Review.
-
Using human induced pluripotent stem cell-derived cardiomyocytes to understand the mechanisms driving cardiomyocyte maturation.Front Cardiovasc Med. 2022 Aug 12;9:967659. doi: 10.3389/fcvm.2022.967659. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36061558 Free PMC article.
Cited by
-
Comprehensive promotion of iPSC-CM maturation by integrating metabolic medium with nanopatterning and electrostimulation.Nat Commun. 2025 Mar 21;16(1):2785. doi: 10.1038/s41467-025-58044-6. Nat Commun. 2025. PMID: 40118846 Free PMC article.
-
AMPK activator-treated human cardiac spheres enhance maturation and enable pathological modeling.Stem Cell Res Ther. 2023 Nov 8;14(1):322. doi: 10.1186/s13287-023-03554-7. Stem Cell Res Ther. 2023. PMID: 37941041 Free PMC article.
-
Sexual Dimorphism in Cardiometabolic Diseases: The Role of AMPK.Int J Mol Sci. 2023 Jul 26;24(15):11986. doi: 10.3390/ijms241511986. Int J Mol Sci. 2023. PMID: 37569362 Free PMC article. Review.
-
Promotion of maturation of human pluripotent stem cell-derived cardiomyocytes via treatment with the peroxisome proliferator-activated receptor alpha agonist Fenofibrate.Stem Cells Transl Med. 2024 Aug 16;13(8):750-762. doi: 10.1093/stcltm/szae029. Stem Cells Transl Med. 2024. PMID: 38946019 Free PMC article.
-
Cardiac Organoids to Model and Heal Heart Failure and Cardiomyopathies.Biomedicines. 2021 May 18;9(5):563. doi: 10.3390/biomedicines9050563. Biomedicines. 2021. PMID: 34069816 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

