Adeno-associated virus serotype 9 antibodies in patients screened for treatment with onasemnogene abeparvovec
- PMID: 33768131
- PMCID: PMC7973120
- DOI: 10.1016/j.omtm.2021.02.014
Adeno-associated virus serotype 9 antibodies in patients screened for treatment with onasemnogene abeparvovec
Abstract
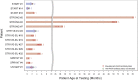
Spinal muscular atrophy is a progressive, recessively inherited monogenic neurologic disease, the genetic root cause of which is the absence of a functional survival motor neuron 1 gene. Onasemnogene abeparvovec (formerly AVXS-101) is an adeno-associated virus serotype 9 vector-based gene therapy that delivers a fully functional copy of the human survival motor neuron gene. We report anti-adeno-associated virus serotype 9 antibody titers for patients with spinal muscular atrophy when they were screened for eligibility in the onasemnogene abeparvovec clinical trials (intravenous and intrathecal administration) and managed access programs (intravenous). Through December 31, 2019, 196 patients and 155 biologic mothers were screened for anti-adeno-associated virus serotype 9 binding antibodies with an enzyme-linked immunosorbent assay. Of these, 15 patients (7.7%) and 23 biologic mothers (14.8%) had titers >1:50 on their initial screening tests. Eleven patients (5.6%) had elevated titers on their final screening tests. The low percentage of patients with exclusionary antibody titers indicates that most infants with spinal muscular atrophy type 1 should be able to receive onasemnogene abeparvovec. Retesting may identify patients whose antibody titers later decrease to below the threshold for treatment, and retesting should be considered for patients with anti-adeno-associated virus serotype 9 antibody titers >1:50.
Keywords: Adeno-associated virus serotype 9; antibody titers; clinical trials; enzyme-linked immunosorbent assay; gene replacement therapy; gene therapy; managed access programs; onasemnogene abeparvovec; spinal muscular atrophy; survival motor neuron.
© 2021 The Authors.
Conflict of interest statement
J.W.D. reports financial relationships with Novartis Gene Therapies, Inc., Affinia Therapeutics, Biogen, Cytokinetics, Ionis, Pfizer, Roche, and Sarepta, and royalties from Athena Diagnostics. R.S.F. has served as an advisor to Novartis Gene Therapies, Inc., Ionis, Biogen, and Roche, and received licensing fees from the Children’s Hospital of Philadelphia related to development of the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders scale. E.M. has received personal compensation for clinical trial consulting and serving on scientific advisory boards from Novartis Gene Therapies, Inc. K.J.S. has received personal compensation from Novartis Gene Therapies, Inc., for serving as an advisory board member, and from Biogen for serving as a visiting professor; and has received research support from Novartis Gene Therapies, Inc., and Biogen-sponsored clinical trials. M.M. is an employee of Novartis Gene Therapies, Inc. R.v.O. and S.T.-W. are employees of Novartis Gene Therapies, Inc., and own Novartis stock or other equities. J.R.M. declares no competing interests.
Figures
References
-
- Al-Zaidy S.A., Mendell J.R. From clinical trials to clinical practice: practical considerations for gene replacement therapy in SMA type 1. Pediatr. Neurol. 2019;100:3–11. - PubMed
-
- Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. - PubMed
-
- Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

