Spike vs nucleocapsid SARS-CoV-2 antigen detection: application in nasopharyngeal swab specimens
- PMID: 33768365
- PMCID: PMC7993413
- DOI: 10.1007/s00216-021-03298-4
Spike vs nucleocapsid SARS-CoV-2 antigen detection: application in nasopharyngeal swab specimens
Abstract
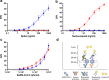
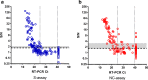
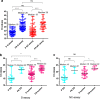
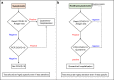
Public health experts emphasize the need for quick, point-of-care SARS-CoV-2 detection as an effective strategy for controlling virus spread. To this end, many "antigen" detection devices were developed and commercialized. These devices are mostly based on detecting SARS-CoV-2's nucleocapsid protein. Recently, alerts issued by both the FDA and the CDC raised concerns regarding the devices' tendency to exhibit false positive results. In this work, we developed a novel alternative spike-based antigen assay, comprising four high-affinity, specific monoclonal antibodies, directed against different epitopes on the spike's S1 subunit. The assay's performance was evaluated for COVID-19 detection from nasopharyngeal swabs, compared to an in-house nucleocapsid-based assay, composed of novel antibodies directed against the nucleocapsid. Detection of COVID-19 was carried out in a cohort of 284 qRT-PCR positive and negative nasopharyngeal swab samples. The time resolved fluorescence (TRF) ELISA spike assay displayed very high specificity (99%) accompanied with a somewhat lower sensitivity (66% for Ct < 25), compared to the nucleocapsid ELISA assay which was more sensitive (85% for Ct < 25) while less specific (87% specificity). Despite being outperformed by qRT-PCR, we suggest that there is room for such tests in the clinical setting, as cheap and rapid pre-screening tools. Our results further suggest that when applying antigen detection, one must consider its intended application (sensitivity vs specificity), taking into consideration that the nucleocapsid might not be the optimal target. In this regard, we propose that a combination of both antigens might contribute to the validity of the results. Schematic representation of sample collection and analysis. The figure was created using BioRender.com.
Keywords: Antigen; Nasopharyngeal swab specimens; Nucleocapsid; SARS-CoV-2; Spike; TRF-ELISA.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. Author correction: A new coronavirus associated with human respiratory disease in China. Nature. 2020;580(7803):E7. doi: 10.1038/s41586-020-2202-3. - DOI - PMC - PubMed
-
- Current molecular and antigen tests with FDA EUA Status. https://www.centerforhealthsecurity.org/covid-19TestingToolkit/molecular.... Accessed Feb 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

