Immunogenicity of the BNT162b2 vaccine in frail or disabled nursing home residents: COVID-A study
- PMID: 33768521
- PMCID: PMC8250586
- DOI: 10.1111/jgs.17153
Immunogenicity of the BNT162b2 vaccine in frail or disabled nursing home residents: COVID-A study
Abstract
Background/objectives: The safety and immunogenicity of the BNT162b2 coronavirus disease 2019 (COVID-19) vaccine in older adults with different frailty and disability profiles have not been well determined. Our objective was to analyze immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in older adults across frailty and disability profiles.
Design: Multicenter longitudinal cohort study.
Setting and participants: A total of 134 residents aged ≥65 years with different frailty and disability profiles in five long-term care facilities (LTCFs) in Albacete, Spain.
Intervention and measurements: Residents were administered two vaccine doses as per the label, and antibody levels were determined 21.9 days (SD 9.3) after both the first and second dose. Functional variables were assessed using activities of daily living (Barthel Index), and frailty status was determined with the FRAIL instrument. Cognitive status and comorbidity were also evaluated.
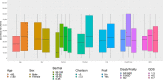
Results: Mean age was 82.9 years (range 65-99), and 71.6% were female. The mean antibody titers in residents with and without previous COVID-19 infection were 49,878 AU/ml and 15,274 AU/ml, respectively (mean difference 34,604; 95% confidence interval [CI]: 27,699-41,509). No severe adverse reactions were observed, after either vaccine dose. Those with prevaccination COVID-19 had an increased antibody level after the vaccine (B = 31,337; 95% CI: 22,725-39,950; p < 0.001). Frailty, disability, older age, sex, cognitive impairment, or comorbidities were not associated with different antibody titers.
Conclusions: The BNT162b2 mRNA COVID-19 vaccine in older adults is safe and produces immunogenicity, independently of the frailty and disability profiles. Older adults in LTCFs should receive a COVID-19 vaccine.
Keywords: BNT162b2 vaccine; COVID-19; disability; frailty; immunogenicity; older adults.
© 2021 The American Geriatrics Society.
Conflict of interest statement
All authors declare that there are no conflicts of interest, except. V.M.L. declares no conflict of interest according to the ICMJE Uniform Requirements but discloses the following financial relationship: CEO and shareholder of HepaPredict AB; co‐founder and chairman of the board PersoMedix AB; consultancy work for Enginzyme AB. JS declares his conflict at
Figures
References
-
- Comas‐Herrera A, Zalakain J, Lemmon E, et al. Mortality associated with COVID‐19 in care homes: international evidence. Article in LTCcovid.org, International Long‐Term Care Policy Network, CPEC‐LSE, 1st February 2021. https://ltccovid.org/2020/04/12/mortality-associated-with-covid-19-outbr....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

