A Cavity-Tailored Metal-Organic Cage Entraps Gases Selectively in Solution and the Amorphous Solid State
- PMID: 33768657
- PMCID: PMC8251750
- DOI: 10.1002/anie.202102095
A Cavity-Tailored Metal-Organic Cage Entraps Gases Selectively in Solution and the Amorphous Solid State
Abstract
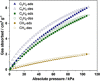
Here we report the subcomponent self-assembly of a truxene-faced Zn4 L4 tetrahedron, which is capable of binding the smallest hydrocarbons in solution. By deliberately incorporating inward-facing ethyl groups on the truxene faces, the resulting partially-filled cage cavity was tailored to encapsulate methane, ethane, and ethene via van der Waals interactions at atmospheric pressure in acetonitrile, and also in the amorphous solid state. Interestingly, gas capture showed divergent selectivities in solution and the amorphous solid state. The selective binding may prove useful in designing new processes for the purification of methane and ethane as feedstocks for chemical synthesis.
Keywords: gas adsorption; gas encapsulation; host-guest chemistry; metal-organic cage; supramolecular chemistry.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- None
-
- Service R. F., Science 2014, 346, 538–541; - PubMed
-
- Yeh S., Energy Policy 2007, 35, 5865–5875.
-
- Barnett B. R., Gonzalez M. I., Long J. R., Trends Chem. 2019, 1, 159–171.
-
- Zhang X., Chen Z., Liu X., Hanna S. L., Wang X., Taheri-Ledari R., Maleki A., Li P., Farha O. K., Chem. Soc. Rev. 2020, 49, 7406–7427. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

