Cell-type specific expression and behavioral impact of galanin and GalR1 in the locus coeruleus during opioid withdrawal
- PMID: 33768673
- PMCID: PMC8376771
- DOI: 10.1111/adb.13037
Cell-type specific expression and behavioral impact of galanin and GalR1 in the locus coeruleus during opioid withdrawal
Abstract
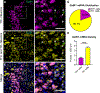
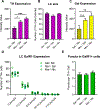
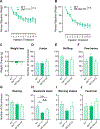
The neuropeptide galanin is reported to attenuate opioid withdrawal symptoms, potentially by reducing neuronal hyperactivity in the noradrenergic locus coeruleus (LC) via galanin receptor 1 (GalR1). We evaluated this mechanism by using RNAscope in situ hybridization to characterize GalR1 mRNA distribution in the dorsal pons and to compare galanin and GalR1 mRNA expression in tyrosine hydroxylase-positive (TH+) LC cells at baseline and following chronic morphine or precipitated withdrawal. We then used genetically altered mouse lines and pharmacology to test whether noradrenergic galanin (NE-Gal) modulates withdrawal symptoms. RNAscope revealed that, while GalR1 signal was evident in the dorsal pons, 80.7% of the signal was attributable to TH- neurons outside the LC. Galanin and TH mRNA were abundant in LC cells at baseline and were further increased by withdrawal, whereas low basal GalR1 mRNA expression was unaltered by chronic morphine or withdrawal. Naloxone-precipitated withdrawal symptoms in mice lacking NE-Gal (GalcKO-Dbh ) were largely similar to WT littermates, indicating that loss of NE-Gal does not exacerbate withdrawal. Complementary experiments using NE-Gal overexpressor mice (NE-Gal OX) and systemic administration of the galanin receptor agonist galnon revealed that increasing galanin signaling also failed to alter behavioral withdrawal, while suppressing noradrenergic transmission with the alpha-2 adrenergic receptor agonist clonidine attenuated multiple symptoms. These results indicate that galanin does not acutely attenuate precipitated opioid withdrawal via an LC-specific mechanism, which has important implications for the general role of galanin in regulation of somatic and affective opioid responses and LC activity.
Keywords: GalR1; galanin; locus coeruleus; noradrenergic; opioid; withdrawal.
© 2021 Society for the Study of Addiction.
Conflict of interest statement
Figures



References
-
- Lang R, Gundlach AL, Holmes FE, et al. Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity. Pharmacol Rev 2015;67(1):118–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

