Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation
- PMID: 33769101
- PMCID: PMC8576366
- DOI: 10.1152/physrev.00022.2020
Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation
Abstract
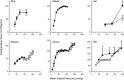
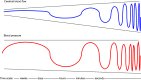
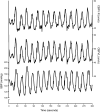
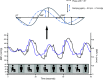
Brain function critically depends on a close matching between metabolic demands, appropriate delivery of oxygen and nutrients, and removal of cellular waste. This matching requires continuous regulation of cerebral blood flow (CBF), which can be categorized into four broad topics: 1) autoregulation, which describes the response of the cerebrovasculature to changes in perfusion pressure; 2) vascular reactivity to vasoactive stimuli [including carbon dioxide (CO2)]; 3) neurovascular coupling (NVC), i.e., the CBF response to local changes in neural activity (often standardized cognitive stimuli in humans); and 4) endothelium-dependent responses. This review focuses primarily on autoregulation and its clinical implications. To place autoregulation in a more precise context, and to better understand integrated approaches in the cerebral circulation, we also briefly address reactivity to CO2 and NVC. In addition to our focus on effects of perfusion pressure (or blood pressure), we describe the impact of select stimuli on regulation of CBF (i.e., arterial blood gases, cerebral metabolism, neural mechanisms, and specific vascular cells), the interrelationships between these stimuli, and implications for regulation of CBF at the level of large arteries and the microcirculation. We review clinical implications of autoregulation in aging, hypertension, stroke, mild cognitive impairment, anesthesia, and dementias. Finally, we discuss autoregulation in the context of common daily physiological challenges, including changes in posture (e.g., orthostatic hypotension, syncope) and physical activity.
Keywords: Alzheimer’s disease; hypertension; microcirculation; neurovascular coupling; stroke.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


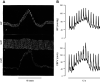
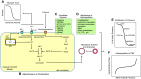

References
-
- Willis T. Cerebri Anatome: Cui Accessit Nervorum Descriptio Et Usus. London: James Flesher, Joseph Martyn and James Allestry, 1664.
-
- Monro A. Observations on the Structure and Functions of the Nervous System. Edinburgh, Scotland: William Creech, 1783.
-
- Hill L. The Physiology and Pathology of the Cerebral Circulation. An Experimental Research. London: J&A Churchill, 1896.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

